1. Organoid and organ chips
Organoids are three-dimensional structures generated by pluripotent or adult stem cells that can reproduce the structure, function and tissue properties of human organs, and are widely used in personalized medicine and preclinical drug testing.
Common organoid culture methods include submerged culture, gas-liquid interaction culture and co-culture.
An organ-on-a-chip (OOC) is a cell culture device fabricated by microfluidics, usually consisting of a micro-perfused chamber with multilayered cellular structures, tissue interfaces, a physicochemical microenvironment, and a vascular-like circulatory system.
It mimics the physiological functions of human organs and is a miniature cell culture system.
The working principle of organ chips is to reproduce the complex environment in the body by simulating physiological conditions such as fluid shear, dynamic mechanical stress, concentration gradient, etc., and then study the interaction of cells, tissues and blood, and observe the pathophysiological responses under different stimuli, so as to provide more realistic and reliable experimental data.
2. Organ chips and their applications
1) Respiratory Organ Chip
Gas exchange in the lungs is mainly through the expansion and contraction of the alveoli to increase the gas exchange area, and the alveoli, as the main site of gas exchange, is also the functional unit of the lungs.
The research team has developed a multifunctional microdevice based on a microfluidic chip that is capable of reproducing the structure of the air-blood barrier at the alveolar-capillary interface and realizing its core functions, with the ability to mimic the basic functional units of the human lung.
The device works as follows: the core of the device consists of two tightly connected microchannels at the top and bottom, separated by a 10-μm-thick porous flexible film of polydimethylsiloxane (PDMS).
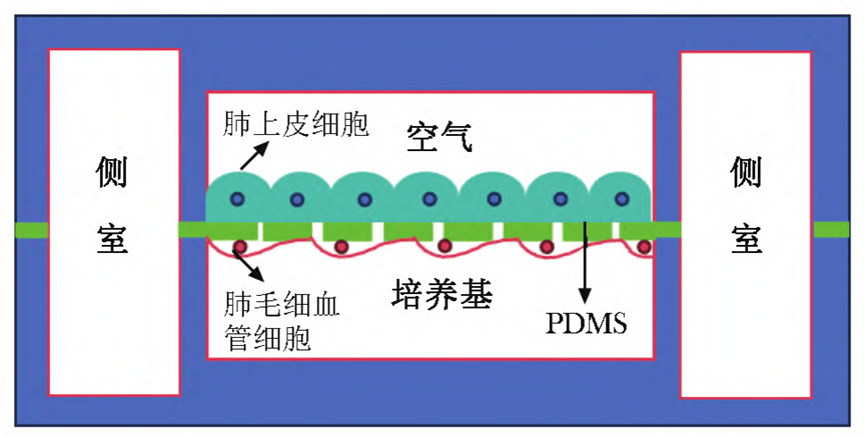
After the surface of the film was coated with fibronectin or collagen, human alveolar epithelial cells and human microvascular endothelial cells were cultured on the upper and lower sides, respectively.
After the cells have grown and become confluent, air is introduced into the upper chamber and the medium is retained in the lower chamber, forming an air-liquid interface.
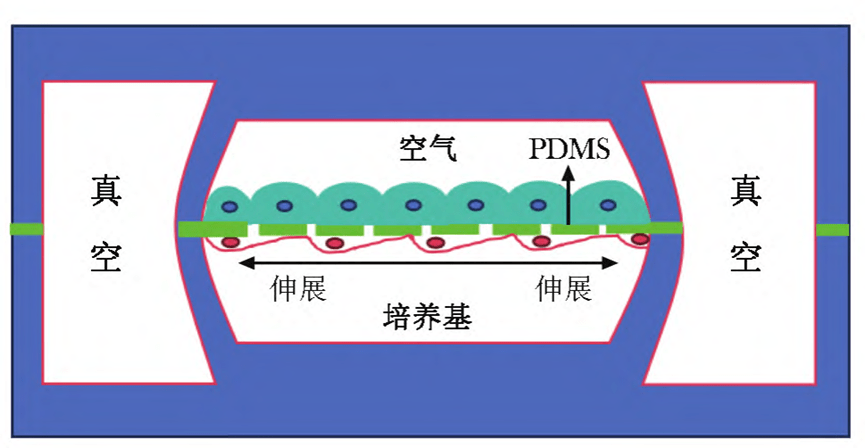
When the side chamber is under vacuum, the PDMS film undergoes elastic deformation, driving the cells on the film to stretch;
When air pressure is restored, the elastic action of the film restores the cells to their original state, thus simulating the dynamic mechanical changes at the alveolar-capillary interface induced by respiratory movements.
2) Circulatory System Organ Chip
Cardiomyocytes do not regenerate, and cardiotoxicity of drugs is critical in drug evaluation.
The development of more accurate cardiac models is especially critical in order to better model the disease and predict the cardiotoxicity of drugs.
Cardiac microarrays have received much attention in recent years as an in vitro model that integrates multiple factors such as controllable physiological, biochemical and mechanical forces.
Existing cardiac microarrays typically consist of four main components: microfluidic structures, cellular tissues and substrates, environmental control systems (e.g., oxygen gradients, drug delivery, mechanical stimulation), and analytical components (e.g., on-line biochemical sensors and electrodes).
These cells are usually derived from adult donor cell-derived induced pluripotent stem cells that are cultured to generate cardiomyocytes.
Based on this technology, a variety of cardiac OOC platforms have been developed that focus on mimicking the biological functions of the heart by co-culturing different types of cells, such as cardiomyocytes, endothelial cells, and cardiac fibroblasts.
Also, cardiac microarrays are widely used to assess the cardiotoxicity of anticancer drugs.
3) Digestive System Organ Chip
Drug-induced liver injury is a major cause of drug failure in preclinical and clinical trials, and a common cause of drug withdrawal after marketing.
Liver organoids usually use primary human hepatocytes or cell lines, but cell function declines over time in culture.
To address this issue, the study was able to partially restore hepatocyte function through co-culture with astrocytes, fibroblasts, and endothelial cells, as well as hemoperfusion.
Due to the unique role of the liver in drug metabolism and toxic effects, liver microarrays are often combined with other organ microarrays as a key model for multi-organ microarray studies.
The gut is the body's primary organ for nutrient digestion, absorption, and metabolism, as well as a key area for host interaction with commensal flora and mucosal immunity.
By combining microfluidic technology and simulated force action, the intestinal organ chip is able to more realistically reproduce the anatomy and physiology of the intestinal tract.
On this basis, the researchers have developed intestinal disease models such as Crohn's disease and ulcerative colitis, and have established a series of intestinal infection models using intestinal organ chips for studying the pathogenic mechanisms of pathogens.
4) Nervous system - brain/blood-brain barrier chip
The complex structure and function of the human brain, especially the existence of the blood-brain barrier, makes the development of neurological drugs uniquely challenging.
The blood-brain barrier increases the difficulty of efficacy prediction by selectively controlling drug entry into the central nervous system.
Therefore, the research and development of brain chips mainly focuses on simulating the blood-brain barrier function, and reconstructing the human blood-brain barrier with physiological functions through precise three-dimensional arrangement of cells, inter-cell interactions, and organ-specific mechanical and biochemical gradients.
This provides an important platform for testing whether drugs against neurological disorders can cross the blood-brain barrier and act at specific targets.
Blood-brain barrier microarray models have been widely used in neuropathology for modeling various types of diseases, helping to gain insights into the pathogenesis and progression of diseases, identifying potential therapeutic targets, and evaluating the therapeutic efficacy of new drugs.
Currently, established disease models include Alzheimer's disease, Parkinson's disease, and neuroinflammatory responses.
5) Urinary System - Kidney Chip
The kidney plays a key role in the body's metabolism, excretion, and reabsorption processes and is a major target organ for drug toxicity.
Drug-induced renal injury is an important factor in assessing drug toxicity and determining safe doses.
The renal proximal tubule is the most susceptible site to drug toxicity; therefore, proximal tubule OOC modeling is important in the prediction of drug toxicity.
Accuracy of partial organ microarrays in drug toxicity testing is superior to traditional preclinical models.
As a result, researchers can re-assess the toxicity of some drugs that did not pass preclinical trials, which could lead to new discoveries.
In addition, there is a close interaction between the liver and kidneys as the body's main detoxification organs.
The establishment of liver-kidney organ microarrays and their drug metabolism data suggest that multi-organ synergistic platforms are more sensitive to drug toxicity responses compared to single-organ microarrays, which makes them show higher potential in replacing animal models for drug toxicity screening.
6) Other chips
With the continuous advancement of organ chip technology, researchers are demanding more types and functions of organ chips.
Skin chips, bone chips, muscle chips, retina chips and tumor chips have been introduced, laying the foundation for the construction of organ chip platforms and multi-organ chip joint platforms that are closer to the physiological and pathological states of the human body.
On this basis, combining tumor microarrays with other organ microarrays enables a more systematic study of how tumors invade and metastasize through blood vessels and lymphatic vessels and other complex pathological processes and physiological responses.
With the gradual improvement of the functions of individual organ chips, when these chips are equipped with key features that mimic the corresponding organs, they can be combined into a joint multi-organ chip platform, which can also be referred to as a human chip.
By simulating the in vivo environment such as vascular perfusion through microfluidic technology and interconnecting different single-organ chips, it is able to reproduce the complex dynamic interactions between organs and provide a drug delivery, distribution and absorption model closer to the physiological state.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1