1. Conventional methods for the preparation of lipid-based nanoparticles
The formation of lipid nanoparticles is a self-assembly process, and their final morphology is determined by a combination of thermodynamic states, lipid-lipid interaction forces, and molecular geometry.
Conventional methods for the production of lipid nanoparticles can be categorized according to the physical mechanisms controlling the kinetics of self-assembly, with the most widely used being processes based on mechanical homogenization, solvent dilution, or decontaminant removal.
1) Mechanical homogenization
In the case of mechanical homogenization, large polydisperse multiciliated vesicles (MLVs) are first prepared using techniques such as lipid film hydration, and then the resulting emulsion is subjected to a high-pressure gradient or shear to rupture the large vesicles, exposing the hydrophobic membrane core to the aqueous phase and allowing the resulting fragments to re-form into smaller monociliated vesicles.
An advantage of mechanical disruption is that the initial MLV solution is an aqueous solution, thus eliminating the need for organic solvents in the preparation process.
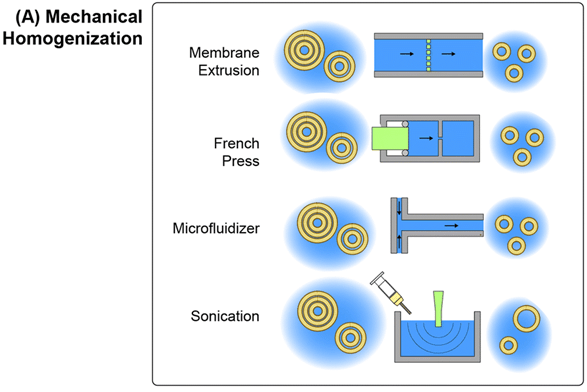
Ultrasound is a common mechanistic technique for the synthesis of small-volume liposomes, where ultrasonic energy can produce cavitation in the lipid solution, leading to high transmembrane pressure gradients that can rupture multifibrillar layer vesicles.
2) Descaler Removal
The descaler-based removal method begins with the use of a descaler to stabilize the lipid micelle structure in an aqueous solution.
As with the mechanical disruption method, this preparation avoids the use of solvents in the initial lipid solution.
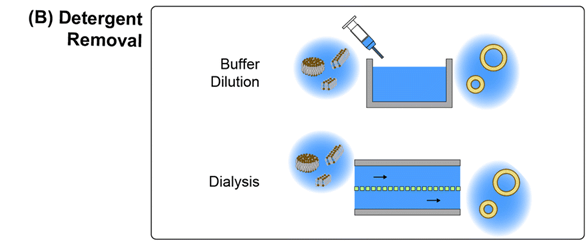
Since descalers are more water soluble than lipids, removal or dilution of the descaler in the surrounding medium results in a rapid decrease in the concentration of descaler in the micelles, which leads to destabilization of the micelles and conversion to spherical vesicles.
Descalers can be removed by dialysis or by rapid dilution of the micelles with an aqueous buffer.
Descaler removal techniques can operate at high flux, but tend to produce larger, more dispersed particles than other techniques, in part because of limited control over the size and morphology of the initial micelle structure.
3) Solvent dilution
Unlike mechanical disruption and descaler removal, formation of lipid nanoparticles by solvent dilution uses lipid solutions dispersed in water-soluble organic solvents such as ethanol.
The solvent dilution process is a flash nanoprecipitation method in which the solubility of the molecules decreases rapidly, thus inducing the particles to precipitate out of the constituent solute.
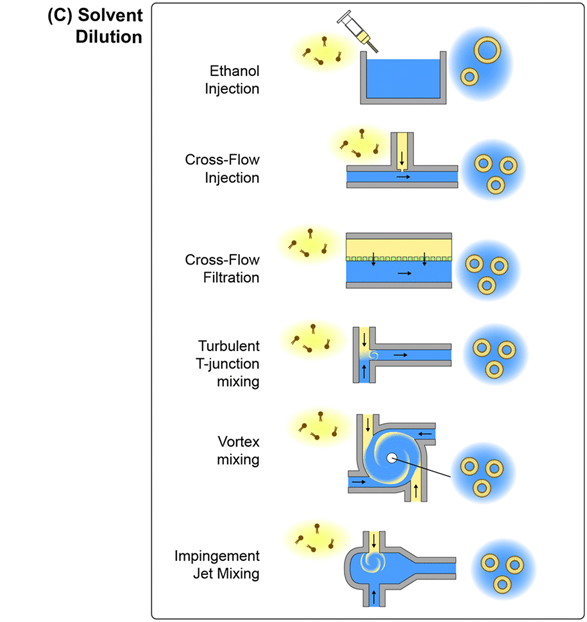
When this technique was first used for the production of lipid-based nanoparticles, ethanol-solubilized lipids were required to be injected into an aqueous buffer, resulting in relatively large (>200 nm) and polydisperse monoamide vesicles.
A cross-flow injection technique was later developed in order to improve the size control of ethanol injection while realizing continuous flow nanoparticle production.
In this method, dissolved lipids are injected into the buffer stream through an orifice, and the orifice size, lipid injection pressure, and buffer flow rate are chosen to improve mixing kinetics.
2. Microfluidics-based synthesis of lipid nanoparticles
Microfluidic methods used for lipid-based nanoparticle synthesis focus on nanoprecipitation by solvent dilution.
In these systems, nanoparticle self-assembly is driven by steep spatial and temporal solubility gradients that are induced by convection-controlled rapid mixing within microchannels, which typically have feature sizes between tens and hundreds of micrometers.
In these systems, mixing is achieved through channel designs that improve mixing performance by optimizing diffusive transport, advective transport, or a combination of both mechanisms.
1) Microfluidic hydrodynamic focusing
The first microfluidic technology used for the synthesis of lipid-based nanoparticles was based on the microfluidic hydrodynamic focusing (MHF) technique.
In this process, the central stream of dissolved lipids is wrapped by a pair of outer aqueous buffer streams that focus the solvent stream into narrow sheets.
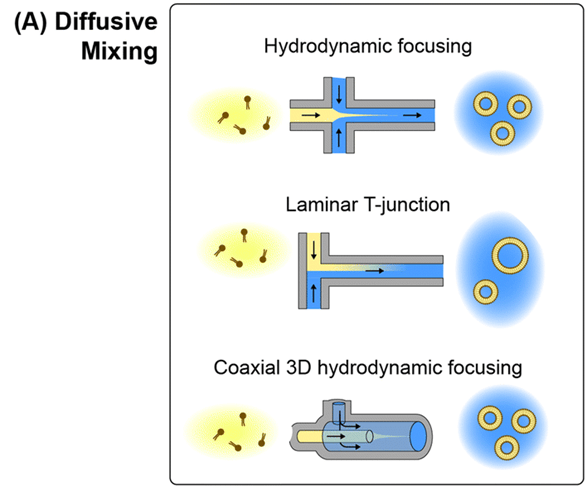
The reduced diffusion length scale of solvent, water and lipid within the laminar mixing zone leads to an increased solubility gradient, which shortens the time scale for the growth of intermediate lipid fragments, thus limiting the size of the resulting vesicles.
Compared to conventional homogenization techniques, the MHF process can yield monociliated lipid nanoparticles with lower polydispersity by passing through the continuous flow mixing zone only once.
2) Microfluidic chaotic advection
In order to overcome the slow mixing speed of laminar T-junction mixers, various microchannel designs capable of manipulating the flow lines within the mixing channel have been explored to induce fast mixing through chaotic advection.
During chaotic advection, mixing in low Reynolds number flows is characterized by the formation of streaks by stretching and folding of merging fluid domains, which promotes rapid diffusion across domain boundaries.
Watercourse geometries used for this purpose include periodic turns, notches, obstructions, or bifurcated flow paths that disrupt linear flow lines.
While both advection and diffusion contribute to mixing in these systems, the mixing rate is largely dependent on the enhancement of the advection mixing process. Such platforms are known as microfluidic chaotic advection (MCA) mixers.
Like the MHF device, the MCA mixer serves to dilute the solvent concentration below the lipid solubilization limit faster than the characteristic growth rate of lipid fragments.
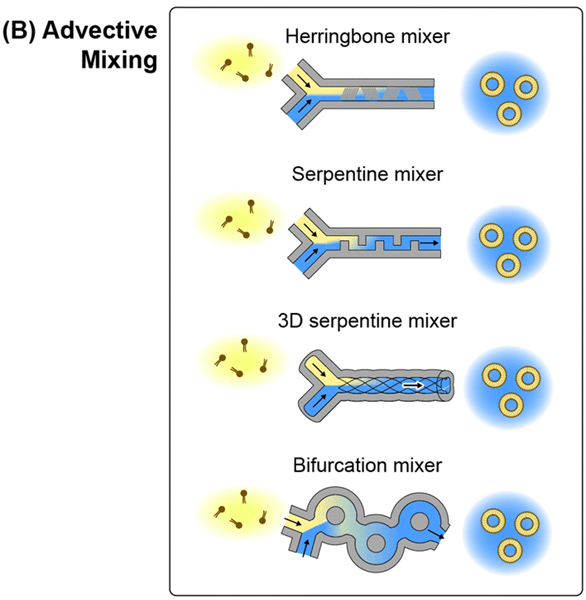
Other MCA channel topologies have also been used for the production of lipid-based nanoparticles.
One approach employs periodic serpentine mixing channels, i.e., curved channels are utilized to induce secondary chaotic Dean vortices in the flow that fold into each other with each change in curvature.
This concept has been applied to synthesize liposomes and LNPs using various serpentine channel designs.
A related technique uses soft lithography to create three sets of intertwined channels, using manually twisted threads to define the channel mold.
3) Combination of flow focusing and advection mixing
The team introduced a vortex focusing technique that combines MHF and MCA in a single chamber to enhance mixing during nanoparticle synthesis.
Unlike conventional vortex mixers that use planar mixing chambers to produce nanoparticles in a turbulent vortex flow, the vortex focusing technique involves injecting dissolved lipids into a conical mixing chamber through an axial inlet while simultaneously injecting an aqueous buffer through a tangential inlet to create a laminar helical flow path of buffer that wraps around the central lipid stream.
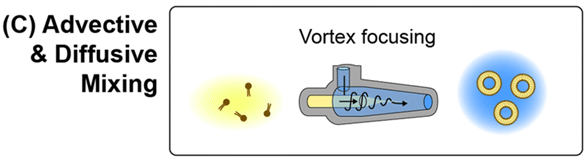
The outer vortex concentrates the lipid solution, reducing the radial diffusion length scale in a manner similar to the MHF technique, while also transferring rotational momentum to the inner flow, leading to interfacial streaking and thus enhanced mixing via chaotic advection.
Similar to MHF, the degree of focusing of the lipid solution can be controlled by adjusting the flow rate ratio.
Using this process, PEGylated liposomes as small as 27 nm can be obtained using neutral lipid mixtures with PDI values below 0.05.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1