Cell types and states vary widely (i.e., cellular heterogeneity), and single-cell analysis has become a popular and indispensable strategy to better explore the unknown characteristics and dynamics of cells and to gain insights into the behavior of tissues, organs, and even whole organisms.
Microfluidic droplet technology can be used to generate high-throughput microdroplets, thus enabling massively parallel studies on single cells.
Although the encapsulation rate of single cells is relatively low using classical microdroplet methods, especially those based on passive droplet generation, the total number of single cells and throughput are much higher than those using other microfluidic single-cell manipulation methods such as microwells and micropatterns.
In addition, microdroplets can encapsulate individual cells in nanoliter droplets, thereby further reducing dilution/cross-contamination/adsorption of substances, improving the exchange efficiency of oxygen, nutrients and metabolites, maintaining cellular activity and biological functions, and facilitating cellular research at the single-cell level.
Meanwhile microfluidic droplet technology enables flexible single-cell manipulation and the integration of different cell research processes, including cell encapsulation, culture, isolation/sorting and assaying, into a single device.
1. Single-cell encapsulation in droplets
Single-cell encapsulation in droplets is achieved primarily through fluid dynamics and surface tension manipulation.
The droplet size is determined by the viscosity of the continuous phase, the interfacial tension, the microchannel size, and the flow rates of the dispersed and continuous phases, with droplet diameters ranging from tens of micrometers to hundreds of micrometers.
The high flow rate and small size of single-cell microdroplets are features that make them suitable for high-throughput single-cell analysis.
However, the encapsulation of single cells is often stochastic, especially when employing passive droplet generation methods typically used for single-cell analysis.
1) Randomized single-cell encapsulation
In the randomized encapsulation method, the number of cells per droplet depends on the initial concentration of the cell suspension and the droplet volume, but will show a highly random distribution in which the proportion of single-cell droplets is low.
Therefore, the control of the number of cells in a single droplet must be further enhanced.
To improve the efficiency of single-cell encapsulation, some researchers have used randomized encapsulation followed by sorting.
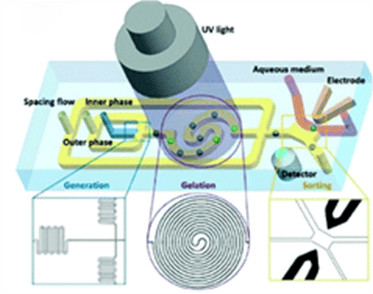
The figure above describes a microfluidic system for the production and sorting of single-cell microcapsules, by which high throughput preparation of microdroplets and microgels can be achieved in a microfluidic device.
Importantly, on-chip sorting electrodes were used to accomplish screening of single-cell microcapsules while transferring them into the culture medium.
They achieved a 16% single-cell encapsulation rate at a cell density of 3.05 × 106 cells per milliliter and a droplet diameter of 50 μm.
After sorting, single-cell encapsulation is increased to over 80%. This approach can be used to integrate droplet gelation, single-cell microgel sorting, and transfer to culture media, resulting in high-throughput analysis at the single-cell level and comprehensive assessment of cellular heterogeneity.
2) Controlled single-cell encapsulation
As mentioned earlier, the results of the process of random encapsulation of cells in suspension into droplets generally follow a Poisson distribution, which typically results in relatively low single-cell encapsulation rates.
To increase the efficiency of single-cell encapsulation, individual cells can be encapsulated into droplets in curved microchannels using an inertial sorting method in which inertial and Dean forces combine to even out cell spacing.
By matching the cell flow cycle to the droplet generation cycle, the number of empty and multicellular droplets can be reduced and the efficiency of single-cell encapsulation can be improved.
The researchers combined spiral and serpentine channels to effectively focus cells and microbeads.
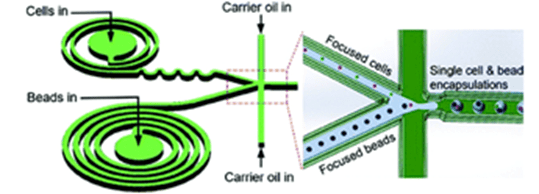
They used this method to encapsulate barcoded microbeads and human mouse cells, and then characterized the heterogeneity of the cells by sequencing.
Using this method, they increased cell utilization by 300% and 40%, respectively, compared to a conventional Drop-seq device and a device used only to focus microbeads.
The results demonstrate that their microfluidic chip improves operational efficiency. This chip design has great potential for realizing efficient single-cell expression profiling.
2. Single-cell encapsulation in hydrogels
In addition to simulating three-dimensional (3D) microenvironments to support cellular activity and function, encapsulation of single cells in microscale hydrogels serves as a good tool for performing cell-independent manipulation and monitoring, which is essential for exploring single cell activity in a living-like microenvironment.
Unlike single-cell-encapsulated droplets, microgels can form three-dimensional network structures whose morphology can be controlled by adjusting the concentration of gel monomers or polymers.
This ensures the supply of oxygen, nutrients and growth factors as well as the timely elimination of cellular metabolic wastes, thus making it possible to culture cells in gel microspheres for extended periods of time.
By applying appropriate chemical or physical methods, the uncured gel in the droplets can be activated and converted into gel microspheres.
Hydrogel microspheres that can be used to produce encapsulated single cells are available in both synthetic and natural polymer materials.
1) Encapsulation using synthetic polymers
In microfluidic droplet technology, synthetic polymers are used as materials to produce cell-encapsulated gel microspheres that can be chemically modified to directly control the cellular microenvironment.
Commonly used synthetic polymers include poly (N-isopropyl acrylamide) and polyethylene glycol diacrylate.
Synthetic polymers with tunable properties in terms of molecular weight, structure and crosslink density can be used to produce gel microspheres with different mechanical properties, generally stronger than natural hydrogels.
Synthetic hydrogel capsules contain a semi-permeable shell of a few micrometers and can be used for multi-step molecular biology assays such as genome amplification.
Generally, synthetic polymers are polymerized with UV light to form gel microspheres for encapsulating cells.
Cell suspensions containing photoinitiators, crosslinkers and monomers were used as the dispersed phase.
After generating microdroplets in the microfluidic chip, the photoinitiator promotes the cross-linking reaction of the monomers under UV irradiation in the presence of a cross-linking agent to produce gel microspheres.
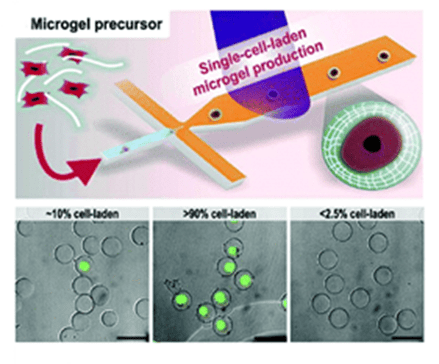
The researchers formed microdroplets encapsulating pluripotent human mesenchymal stem cells or bovine chondrocytes in a microfluidic flow focusing device at an encapsulation speed of 1 kHz using polyethylene glycol diacrylate as the raw material.
Droplets were photocrosslinked through an external UV light source in the downstream channel to prepare cell-loaded microgels and maintain high cellular activity.
The flow cytometer-sorted single-cell microgels (>90% purity) are then mixed with different biocompatible materials (e.g., polyethylene glycol diacrylate and alginate) to make a variety of modular bioinks that can be used for 3D printing to reconstruct the cellular microenvironment at single-cell resolution.
2) Encapsulation using natural polymers
Unlike synthetic polymers, natural polymers are derived from metabolites produced by the organism itself.
In recent years, natural polymers have been widely used for cell encapsulation due to their good biocompatibility and mild polymerization conditions.
Natural polymers used for single-cell encapsulation include sodium alginate, agarose, gelatin, collagen, and hyaluronic acid, with sodium alginate, agarose, and gelatin being the most commonly used.
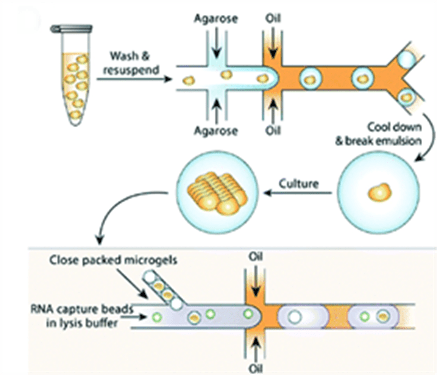
Some researchers have proposed isogenic colony sequencing as a general method for high-throughput analysis of cellular gene expression.
In their method, individual yeast cells are wrapped in low melting point agarose to form agarose droplets that are collected into 50 mL centrifuge tubes and placed on ice to form an agarose microgel.
The agarose microgels were resuspended in a suitable medium for overnight incubation to form individual yeast cell colony microgels, which provide sufficient RNA for deep sequencing of the colonies and reduce the errors that occur in gene expression profiling of individual cells.
Site Search
Recommendations
© 2025. All Rights Reserved. 苏ICP备2022036544号-1