1. Structure of microdroplets
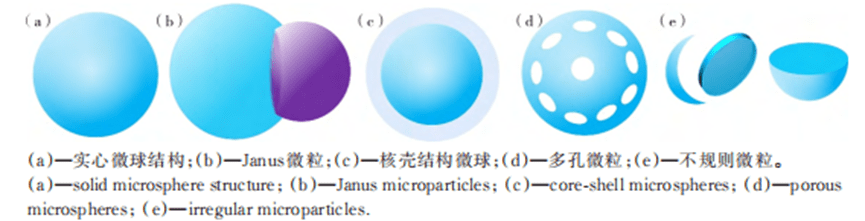
1) Microspheres and microgels
Monodisperse emulsion droplets prepared using T-channel structures, flow-focused structures, and coaxial-focused structures remain spherical due to minimized surface energy. During solidification, individual emulsion droplets can be transformed into polymer microspheres or microgels consisting of polymer chains or cross-linked polymer networks.
These particles are used in a wide range of applications in filling, mechanical support and medicine.
In particular, monodisperse particles with tunable size, excellent mechanical properties and functionality play an important role in biomedical applications such as drug delivery and cell encapsulation.
For example, polymer microspheres or microgels prepared by droplet microfluidics have drug release characteristics, biodistribution, and routes of administration that vary with drug particle size and material composition, enabling flexible drug delivery;
Encapsulating the cells in a microgel provides the cells with a biocompatible, three-dimensional microenvironment that not only protects the cells from external disturbances, but also ensures the supply of water, oxygen, and nutrients that the cells need.
Adjustment of the molecular structure within the microgel alters its mechanical properties, thereby affecting cell migration, proliferation and differentiation.
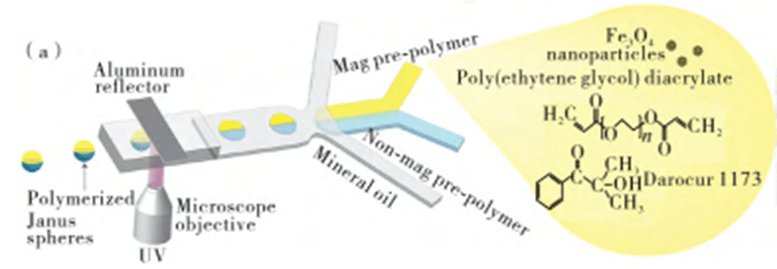
2) Janus particles
Janus particles have two or more separate compartments, each composed of different components, which can synergize or antagonize each other to exhibit unique physicochemical properties, and are often used for special applications such as drug delivery.
Janus droplets as templates can be used to directly synthesize various types of Janus hydrogel particles by polymerization or ionic cross-linking in the dispersed phase through a flow-focused structure combined with droplet microfluidics.
Janus hydrogel particles with superparamagnetism and chemical anisotropy can be self-assembled into two-dimensional chain-like structures using an applied magnetic field.
Homogeneous emulsion droplets can also be synthesized as Janus particles by phase separation.
3) Microcapsules with core-shell structure
Bubbles surrounded by a protective shell are often used for controlled release of substances and particles or microcapsules of nucleoshell structures, usually consisting of solid, liquid or multifunctional materials and capable of acting synergistically or antagonistically through the combination of different components.
With their unique core-shell structure, these microcapsules effectively encapsulate and protect the drug from the external environment.
Depending on the choice of shell layer material, microcapsules can have multiple functions such as controlled release and stimulus response.
By precisely controlling the size and morphology of the homogeneous emulsion droplets, microcapsules with tunable release characteristics can be prepared to improve the stability of drug release.
Single emulsion droplets can be prepared into microcapsules by phase separation and wettability treatment, while the commonly used double T-channel, double cross flow focusing structure and co-axial focusing structure are used to prepare double emulsion droplets and form stable microcapsules by solidifying the shell layer.
Maintaining the stability of the double emulsion during the shell phase curing process allows a wide range of materials to be used as core-shell materials.
Droplet microfluidics and alcoholene photopolymerization enable the preparation of microcapsules with tunable encapsulation, degradation, and thermal properties, and the design of fast-curing droplet microspheres with continuous flow photopatterns for the production of hemispherical particles.
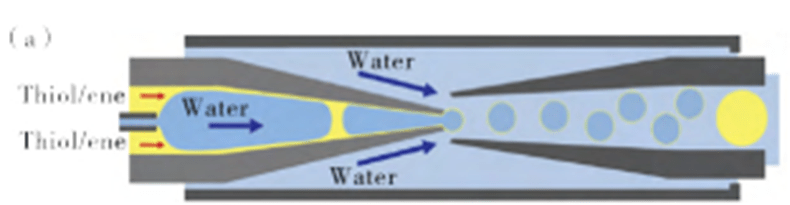
Through continuous exploration, researchers have introduced multiple compartments (which can be located in the nucleus or shell) in emulsion droplets to make the microencapsulation structure more diverse.
For example, microcapsules with multiple cores are synthesized during emulsification using different in-flow separations that can be separated by a solid shell or by forming individual Janus cores inside the shell under UV irradiation.
Tri-emulsified droplets are an effective way to achieve high encapsulation rates of incompatible drugs through the diversity in the selection of core and shell materials.
Triple emulsion droplets with an ultra-thin intermediate layer efficiently encapsulate hydrophobic drugs in polymer microcapsules.
4) Porous particles
Single emulsion droplets were prepared by using T-channel structure, flow focusing structure and co-axial flow focusing structure, which were introduced as precursor droplets into the sacrificial template, and the template was removed after solidification, and the porous particles obtained could be used as carriers for material transportation.
Using microfluidics and selective solvent extraction methods, it is possible to precisely control the formation of endoporous polyelectrolyte particles with diameters ranging from tens of micrometers to hundreds of micrometers, which exhibit a polymer density gradient structure within them, ranging from a dense surface cortex to a hollow core.
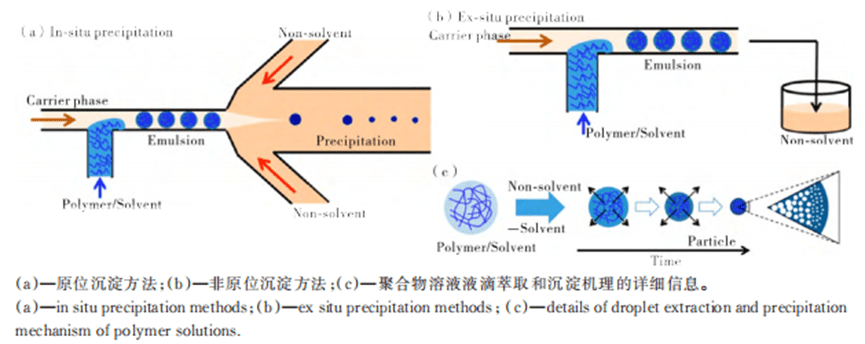
5) Irregular particles
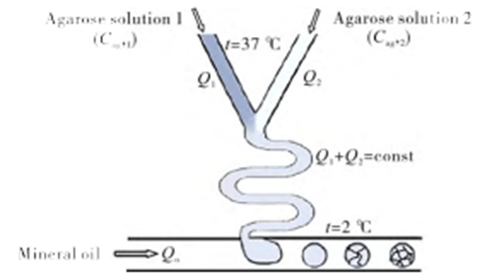
In the preparation of single-emulsion droplets using T-channel structures, flow-focused structures and co-axial flow-focused structures, non-spherical particles can be prepared by adjusting the reaction conditions.
Irregularly shaped particles have special applications, such as crescent-shaped amphiphilic particles.

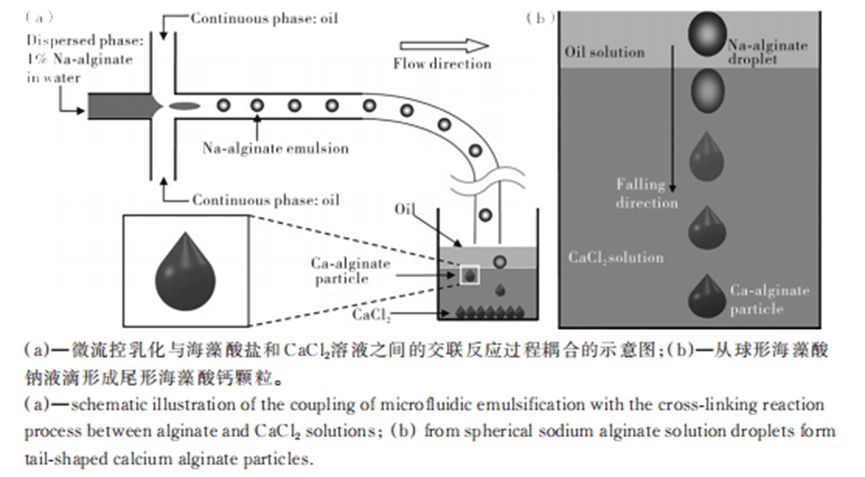
Using an aqueous solution of sodium alginate, which settles in the Ca2+-containing oil phase, droplet- or tail-shaped particles can be produced.
By selectively curing a compartment in a Janus-type emulsion droplet and adjusting the reaction rate, viscosity and Ca2+ concentration, particles of different shapes can be obtained.
In addition, by adjusting the relative values of interfacial tension, dumbbell, acorn or crescent shaped particles can also be prepared.
2. Application of droplet microfluidics to drug discovery and development
1) drug screening
Different drug ingredients can be used to treat a variety of conditions, and selecting the right drug ingredient requires screening.
The biological effects of chemical compounds are closely related to concentration, so determining the appropriate drug concentration requires dose-response screening by pharmacodynamic evaluation.
In a droplet microfluidic system, component concentrations can be precisely controlled by adjusting the relative flow rate.
A method based on the Taylor-Arris dispersion phenomenon has been proposed, by which a parabolic flow profile can be formed within the microfluidic channel, and the concentration distribution of the compounds is transformed from an initial rectangular profile to a Gaussian profile.
This process splits the fluid of compounds and buffers, as well as enzyme and substrate solutions, into droplets that are screened at different points of analysis.
2) Drug encapsulation
Droplet microfluidics can be used for drug encapsulation, especially for encapsulating water-soluble drugs in particles.
When selecting materials, there is a need to ensure compatibility with the drug, especially through W/O or O/W emulsions that can effectively encapsulate hydrophilic or hydrophobic drugs.
The core-shell structure of the particles provides better encapsulation, and the shell layer acts as a barrier to inhibit the diffusion of the drug.
By adjusting the thickness of the shell layer and the grid size, the drug release cycle can be precisely controlled.
The multi-compartmental structure of the particles enables simultaneous encapsulation of multiple drugs for individual microencapsulation and synergistic release, avoiding cross-influence between drugs and thus enabling independent control of drug release.
3) Controlled release of drugs
Sustained release of a drug refers to the gradual release of a drug over a longer period of time and is used in therapeutic regimens where smaller doses are required to improve patient compliance, allowing for long-term, safe and effective drug delivery.
The key to sustained drug release is to control the structure of the particles and the matrix grid size to ensure that the drug is released at a predetermined time.
Sudden drug release, on the other hand, is a controlled release process triggered by an external stimulus, and usually consists of particles of responsive materials that rapidly release the drug in response to a specific stimulus.
These responsive materials can react to external changes, resulting in sudden changes or breaks in the structure of the particles, allowing for rapid drug release.
Temperature-triggered is the most common mechanism of sudden release, whereby a change in temperature induces melting or volume change of the particles to release the drug.
For example, low-temperature cured microcapsules have a solid shell that melts rapidly when the ambient temperature exceeds the melting point of the shell material, resulting in rapid drug release.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1


.png&w=120&h=120&zc=1&q=100)