1. Microvalves
Microvalves are used to regulate fluid flow rates and switching in microfluidic systems.
The ideal microvalve should be low cost, small in size, easy to integrate, have high flow control accuracy, no leakage and fast response time.
Micro-valves control the magnitude and direction of fluid flow, enabling precise, consistent fluid release in chemical or biomedical assays for immediate diagnostics and drug delivery.
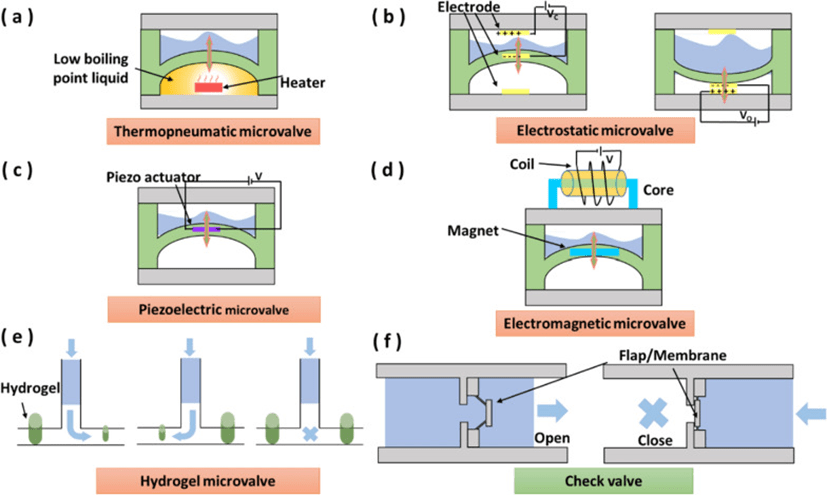
According to the fluid flow control principle, micro valves can be categorized into active and passive types.
Active microvalves utilize external physical fields or chemical stimuli to activate mechanical and non-mechanical moving parts and control flowing fluids.
In contrast, the operating state of a passive microvalve is determined by the fluid, such as the flow direction and the fluid driving pressure.
2. Micropumps
A micropump is a device that continuously conveys a working fluid (liquid or gas) in a precise volume from a reservoir chamber to a specified location.
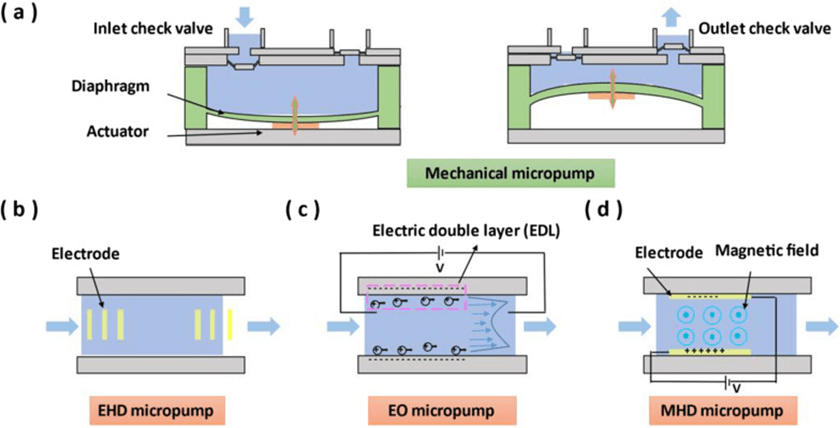
Therefore, the micropump is one of the core components of the microfluidic system and is the power component to realize the fluid supply.
Based on the basic principles, micropumps can be categorized into mechanical micropumps and non-mechanical micropumps. Mechanical micropumps have moving parts for pumping liquids, while non-mechanical micropumps do not.
3. Micromixer chip
Rapid mixing of fluids is critical in microfluidic applications such as drug delivery, sequencing, amplification and biochemical reactions.
However, fluids in microchannels are usually laminar, with Reynolds numbers (Re) less than 1, and are very stable.
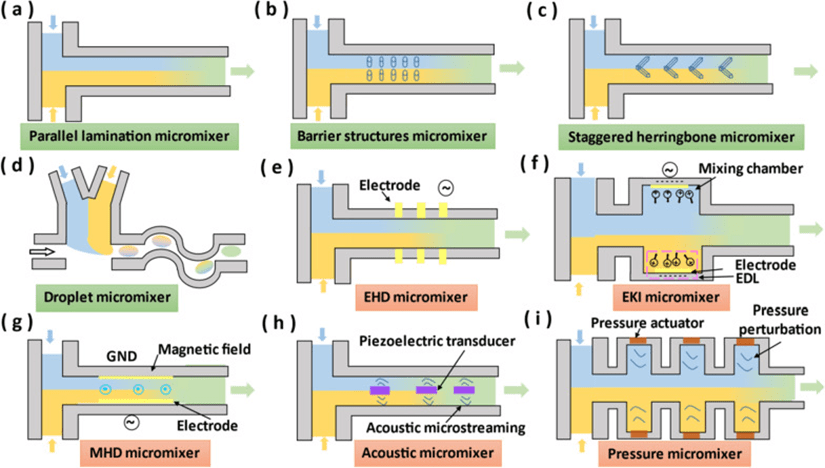
Conventional fluid mixing relies heavily on diffusion and is very inefficient.
Fluids with high Re can form chaotic flows, resulting in shorter mixing lengths and higher mixing efficiencies.
Mixing is mainly dependent on molecular diffusion and chaotic advection. Micromixers can be categorized into passive and active micromixers.
4. Separators
Highly homogeneous clusters of particles, such as beads, cells, vesicles and droplets, are of great importance in the fields of biomedicine, medical research and chemical analysis.
The role of the separator is to separate these target particles from mixtures with different characteristics, and therefore the separator is one of the most important components in a microfluidic system.
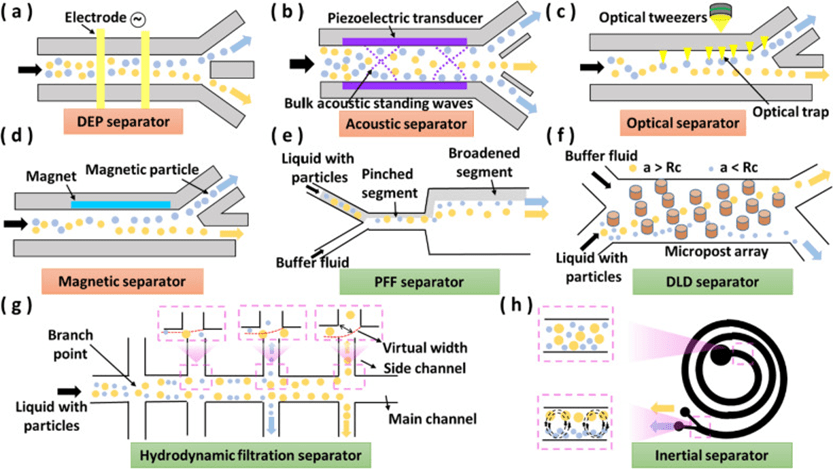
Separators can also be categorized into active and passive types.
Active separators utilize an external physical field to separate particles, while passive separators rely on particle interactions, microchannel geometry, and flow fields to achieve separation.
5. Droplet generator
Droplets in microfluidics are suspended and deformable emulsion microdroplets formed by one liquid (dispersed phase) surrounded by a second immiscible liquid (continuous phase).
The droplet interface acts as a membrane that confines its contents and helps to accurately control the chemical reactions within, and droplets can act as containers for transporting materials and chemical reactions, which prevents sample diffusion and channel wall contamination.
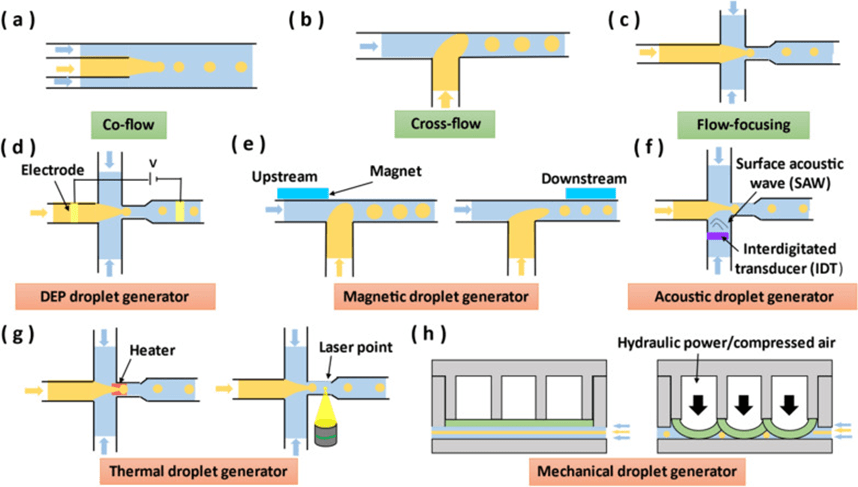
Droplets in microfluidics allow for rapid analysis of very small amounts of reactants in a portable, automated, and inexpensive format compared to the macroscopic operating conditions of traditional chemical reactions.
The nature of the droplet depends on the droplet size and the homogeneity of the sample inside, so generating droplets with controllable size and high dimensional accuracy is important for the accuracy and reproducibility of microfluidic experiments.
6. Gradient generator
In vivo, concentration gradients of biomolecules regulate cellular behavior, such as cell growth and differentiation.
Concentration gradients of chemicals play an important role in many biological phenomena, including inflammation, wound healing, and cancer metastasis.
For chemical analysis, gradient generation can also be applied to drug development and chemical synthesis.
Traditional concentration gradient experiments, such as those performed in multiwell plates, require relatively large volumes of reagents, and the concentration gradient is not precisely and constantly controlled, and the gradient cannot be quickly adjusted.
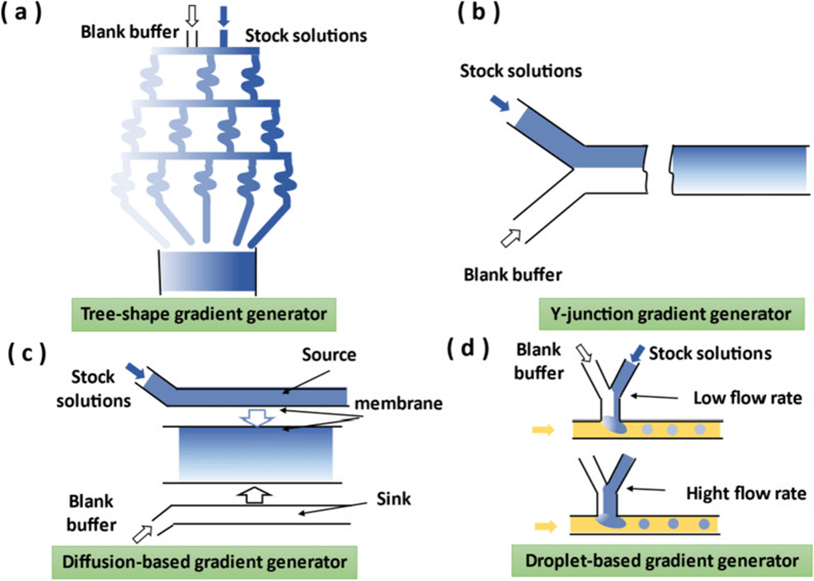
The gradient generator in the microfluidic system requires only a small amount of reagent, which is advantageous for experiments using expensive or scarce reagents.
Gradient generators in microfluidics maintain long-term static stability and fast dynamic response.
Gradient generators mainly include convective, diffusive and droplet generating types.
Convective Gradient Generator In laminar flow, mixable fluids flow side-by-side and are mixed by diffusion.
Diffusive gradient generators typically use membranes or hydrogels to provide high resistance to microchannels, so that solutes in the fluid can only slowly generate gradients by diffusion.
Droplet-type gradient generators are used to generate gradients in droplet arrays where the concentration of the dispersed phase can be adjusted by the inlet flow rate.
7. Captures
Microfluidic capture of individual cells and cell clusters shows great potential in medical and biological research.
In traditional cell studies, cells are studied in larger populations, and measurements only reflect the average of multiple cell responses, potentially ignoring valuable studies of individual cells.
Microfluidic capture technology captures individual cells and clusters of cells at controlled, stable sites, providing a high-throughput, precisely controlled method for analysis at the single-cell level.
Microfluidic traps are generally categorized as contact and non-contact.
Contact traps include notched or raised traps, inertial traps, micro-valve traps, and capillary traps, and non-contact traps mainly include DEP traps, magnetic traps, acoustic traps, and optical traps.
Contact traps utilize a mechanical barrier or obstacle to separate the cell from the fluid, and the cell will stay at the hydrodynamic capture site.
A non-contact trap restricts cell movement through forces created by an external physical field.
8. cell culture
In the life sciences, in vivo cell culture involves animal experiments that are difficult to observe and add to the complexity.
Therefore, the combination of microfluidics and in vitro cell culture has gradually attracted the attention of scientists.
In microfluidic chips, cells are continuously and dynamically supplied with nutrients.
In microfluidic chips, co-cultures can be designed to study the interactions between different kinds of cells.
The co-culture system consists of cultures with direct cell contact and cultures with spatially isolated cells.
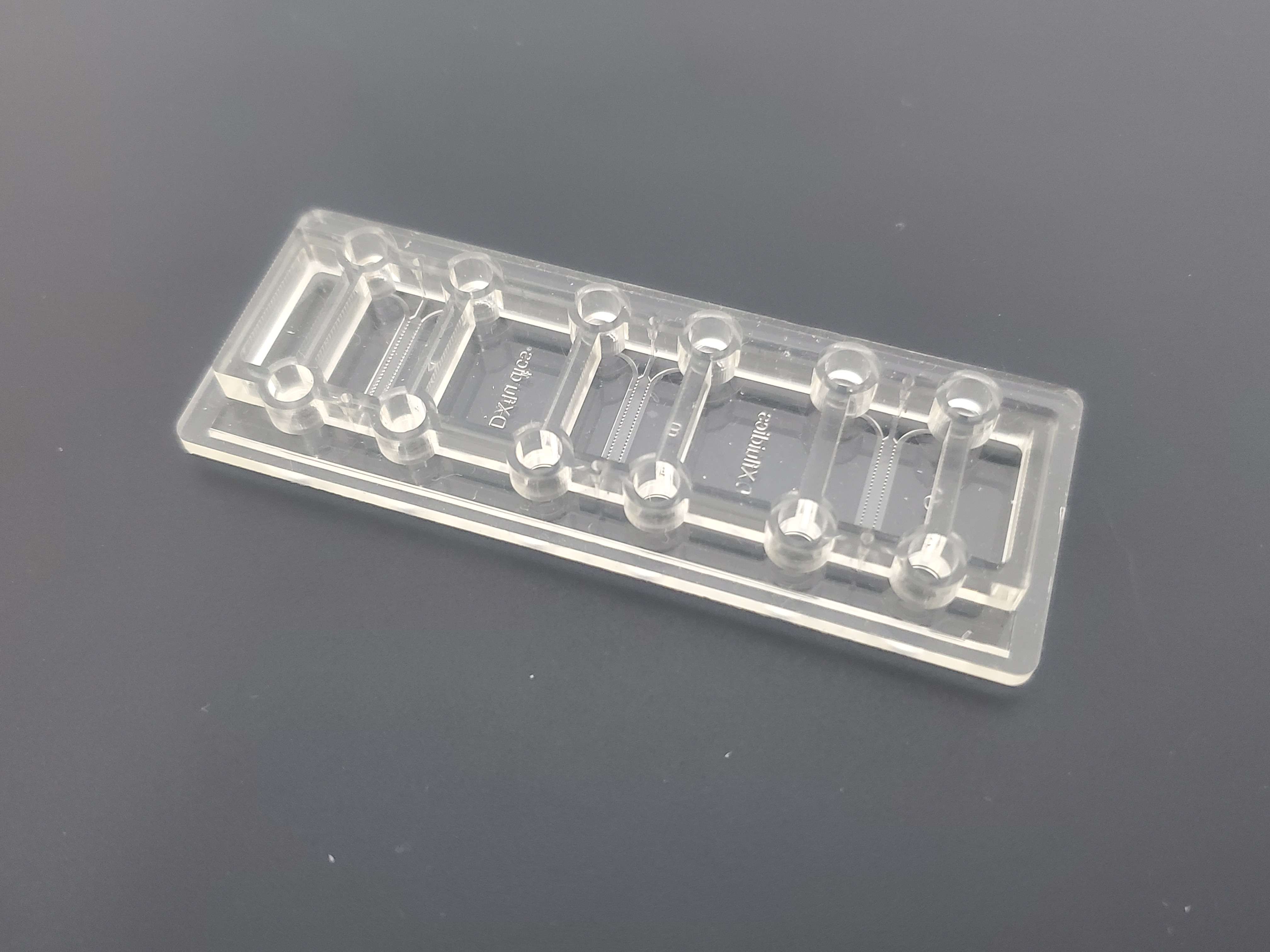
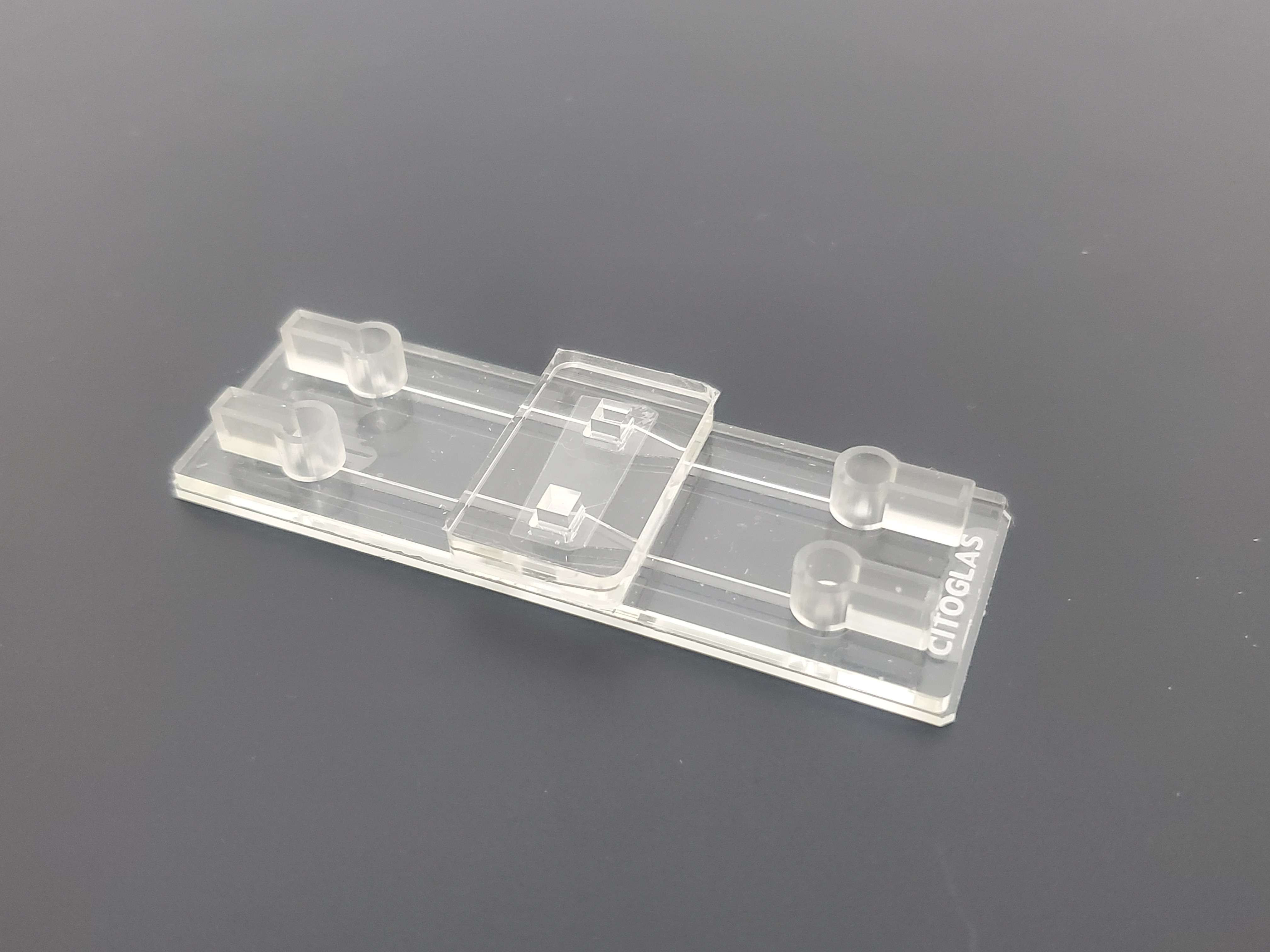
In direct contact co-culture, two or more cells are inoculated at the same interface and are in direct contact to regulate the structure, function and behavior of the cells in the culture chamber.
In indirect co-cultures, where different kinds of cells are usually separated by membranes, cells communicate and influence each other through transmembrane biological factors.
Modular microfluidic co-culture offers better flexibility and applicability than integrated microfluidics.
For example, when studying the co-culture of different tissues under the same conditions, it is difficult to achieve tissue maturation under different conditions and co-culture under the same conditions in an integrated microfluidic chip.
The modular approach allows primary cells to mature on different modules to form different tissues, which are then connected to co-culture different tissues under the same conditions.
In cell culture, in addition to the interactions of co-cultured cells, the microenvironment surrounding the cells is an important factor influencing cell growth, development and repair.
The cellular microenvironment consists of biochemical factors (e.g., soluble factors, extracellular matrix, and gradients) and physical factors (e.g., forces and temperatures that cells can sense).
In contrast to traditional integrated cell culture, microfluidics allows for flexible regulation of biochemical and physical factors in the cellular microenvironment in time and space.
Site Search
Recommendations
© 2025. All Rights Reserved. 苏ICP备2022036544号-1