Cell culture is the process of cell proliferation and development in an artificial environment constructed in vitro. Cells can be grown on the surface of culture vessels (e.g., Petri dishes, microtiter plates) in an adherent wall, or in a suspended state in a substrate.
Traditional methods for detecting cell proliferation and viability include blood cell counting, confocal microscopy imaging, light microscopy combined with fluorescent dye staining, and enzyme-linked immunosorbent assay (ELISA).
These methods have become standard, but are often time-consuming and labor-intensive, and rely on expensive instruments and reagents, making it difficult to achieve high-throughput and efficient operations.
Analytical tools such as fluorescent staining methods are invasive, and not only is the process complicated, but it may also damage the cells and affect subsequent experimental observations.
In contrast, impedance sensing technology has the advantages of being non-invasive, easy to operate and fast to detect.
The technology enables real-time analysis by monitoring impedance changes caused by activities such as cell attachment, proliferation and migration.
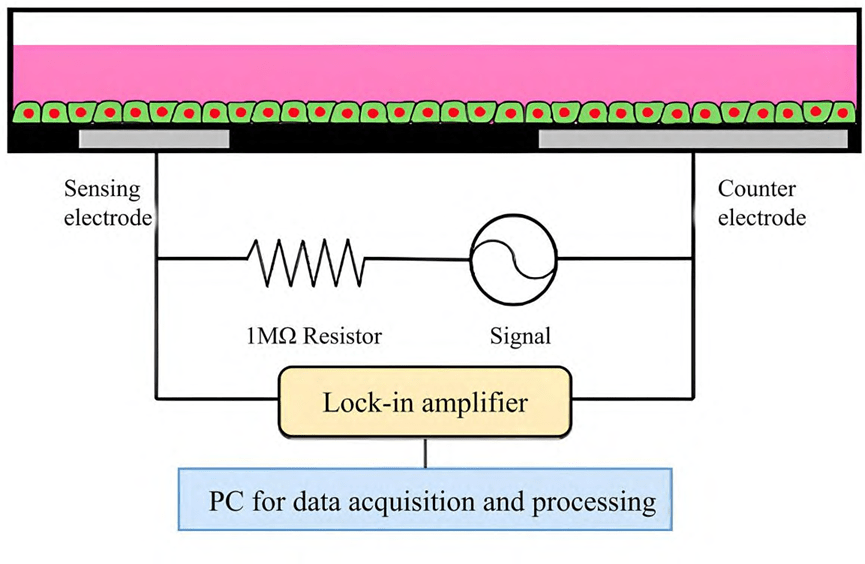
The principle is to apply a sinusoidal voltage of a specific frequency and measure the changes in capacitance and resistance between the electrode and the cell by alternating current to assess the physiological state of the cell. As the cells attach and spread out on the electrode surface, the current is impeded, resulting in an increase in impedance.
Integrating impedance sensors into microfluidic chip platforms enables real-time, efficient, high-throughput cell monitoring. Today, the entire process from sample acquisition to analysis can be integrated on a single chip, and impedance analysis can be accomplished using integrated electrodes to enhance detection efficiency.
A schematic of a typical ECIS system analyzing a cellular system is shown below.
3D cell impedance analysis
Initially, impedance spectroscopy was mainly used for cellular studies in suspensions and two-dimensional (2D) culture systems.
Over time, the limitations of 2D cell culture models have become apparent, while three-dimensional (3D) cell culture models have been widely used in a variety of cellular response studies due to better preservation of the original characteristics of the cells and closer proximity to in vivo physiological states.
It was shown that impedance analysis works in both 2D and 3D cell culture systems for adherent cells and floating cells in suspension.
As 3D cell culture technology matures, this system provides a more physiologically relevant microenvironment for the cells, advancing the use of ECIS (Electrical Impedance Cell Sensing Technology) in 3D cell systems.
Since the complexity of 3D models far exceeds that of 2D models, the characterization process is more difficult and time-consuming.
In particular, the thickness of 3D structures triggers scattering effects, limiting the use of conventional optical techniques, while impedance spectroscopy enables non-invasive, real-time monitoring of 3D culture systems, becoming an alternative to optical microscopy and destructive methods.
In the 2D culture system, impedance detection relies on cell attachment to the electrode surface.
Cells in 2D models lack the necessary cell-cell and cell-matrix interactions due to contact inhibition phenomena, making it difficult to mimic functions and signaling pathways in physiological tissues.
However, primary cells tend to lose their phenotype when cultured in a 2D environment.
The 3D culture system mimics the microenvironment and homeostasis in vivo by encapsulating cells in a scaffold that allows them to grow freely in all directions and interact with their surroundings, thereby reconstructing the interaction of cells with the extracellular matrix.
This 3D model is closer to cell behavior and drug response in vivo, and can more accurately reflect processes such as cell migration, morphogenesis and metabolism, providing a more reliable experimental platform for biomedical research and drug screening.
1) Basic research on impedance in 3D cellular systems
Unlike 2D cell culture, 3D cell culture usually requires the use of cell scaffolds such as agarose gels or matrix gels. These two materials differ in composition and properties, resulting in different impedance assay results.
A single living cell is considered a nonconductor due to the presence of a cell membrane.
As cells aggregate to form tissues, the connections between cells create electrical pathways. When an external electric field is applied in a 3D culture system, an electric current is able to pass through the mixed system of gel and cells.
Dynamic changes in cell proliferation, migration and viability are reflected in the electrical pathways throughout the system by impedance changes.
Although planar electrodes have been used for 3D cell detection, their electric field distribution is not uniform enough to fully characterize the state of 3D cell cultures.
Electrode pair structures have become a more optimal detection solution in 3D culture systems due to their ability to provide a more stable and uniform electric field. Currently, commonly used electrode pair structures include micro-slot electrode pairs and parallel electrode pairs, which enable more efficient impedance monitoring.
2) Research on the application of impedance in 3D cellular systems
a) 3D cell growth, proliferation
A microgroove impedance sensor was developed in recent studies. The sensor captures 3D cells through a microgroove structure with gold electrodes on the microgroove walls for in situ impedance measurements.
With the change in the number of live cells, the impedance between the cells and the matrix gel constructs changed accordingly, and the two were inversely related, thus accurately reflecting the proliferation and apoptosis of 3D cells.
b) 3D cell migration
Cell migration is a complex reaction highly integrated by multi-step molecular processes involved in biological processes such as cancer metastasis, tissue repair, regeneration and wound healing.
The study of cell migration is crucial for parsing the molecular mechanisms behind it. One of the major causes of high cancer mortality is precisely the migration and invasion of tumor cells in 3D tissues.
Quantifying the spatiotemporal dynamics of 3D cell migration or invasion remains challenging.
3D cell culture based on microfluidic platforms can more realistically simulate complex tumor microenvironments (TMEs), provide physiologically and pathologically relevant biological and biophysical information, and effectively bridge the gap between 2D in vitro culture and animal models.
Combining it with impedance monitoring technology enables non-invasive, real-time dynamic monitoring to accurately analyze the migration and invasion process of 3D cells.
c) 3D cell viability, drug screening
Chemotherapy resistance is a major challenge in anticancer treatment, and how to rapidly identify suitable drugs according to the resistance of tumor cells has become an urgent technical bottleneck.
The 3D cell culture system with integrated microfluidic impedance sensing, with the advantages of high throughput, high sensitivity, high accuracy, and low cost, is able to rapidly assess the effects of multiple drugs on tumor cells, providing support for accurately finding the right drugs for different patients.
Compared with traditional 2D culture models, 3D cell models are able to simulate microenvironments that are closer to the human physiological state, significantly improving the accuracy of drug testing, and are therefore widely used in cancer research fields such as antitumor drug screening.
It was shown that chemotherapy toxicity data obtained from impedance-based monitoring were generally consistent with traditional cytotoxicity endpoint assays, further validating the reliability of this technique in drug assessment.
Site Search
© 2024. All Rights Reserved. 苏ICP备2022036544号-1