The two main approaches to constructing artificial cells are top-down and bottom-up. The top-down approach removes non-essential genes and organelles from living cells through biotechnology, resulting in cells with minimal survival functions.
The bottom-up approach, on the other hand, starts with simple inanimate matter and gradually builds bionic structures with cellular functions, avoiding the complexity of cells.
The separation of the internal and external environments is usually simulated using materials similar to cell membranes, and structures such as organelles are assembled inside to mimic the core components of biological cells. These compartments are endowed with specific functions by introducing active substances such as enzymes and genes to support biochemical reactions.
Microstructured systems with the ability to grow, move autonomously and replicate themselves can be assembled in chip-designed microenvironments for highly controlled biochemical reactions. The development of this technology will greatly advance artificial cell research and play a key role in the future development of bottom-up synthetic biology.
1. Main classifications of artificial cells
The interior and exterior of an artificial cell are separated by a barrier that functions similarly to a biological cell membrane. A variety of structural types can be generated when forming the barrier, such as microvesicles, condensates and microdroplets, which have become one of the bases for the classification of artificial cells.
Among them, microvesicles formed by bilayer membranes wrapped around aqueous nuclei are the closest to biological cell morphology and are the common type. Lipid membranes have attracted much attention for their excellent biocompatibility and similarity to cell membranes. Their main building blocks, phospholipids, possess hydrophilic heads and hydrophobic tails and can spontaneously assemble into liposomes in water.
Liposomes are the “gold standard” for artificial cell construction due to their similarity to natural biological membranes and compatibility with membrane proteins. Liposomes are categorized into small unilamellar liposomes (SUVs, 1 μm) and multilamellar liposomes (MLVs) according to their size and number of bilayers.
Of these, GUVs are comparable in size to cells and are a common infrastructure for artificial cells. However, liposome membranes lack proteins and sugars and are not supported by a cytoskeleton.
In contrast, polymer vesicles have better mechanical properties and stability, are more resistant to changes in the external environment, and have greater strength and longevity to protect the contents.
The constituent unit block copolymers can also be customized for their physicochemical properties. However, polymer vesicles are not suitable for the embedding of membrane proteins or molecular channels.
In order to combine the advantages of liposomes and polymer vesicles, lipid/polymer hybrid vesicles have emerged. These vesicles combine the biocompatibility of liposomes with the structural stability of polymers, and their physicochemical properties can be flexibly adjusted to suit different applications.
Advances in microfluidics have accelerated the use of droplet technology. Water-in-oil (W/O) microdroplets comparable to cell size are widely used in artificial cell construction.
The internal environment of such droplets is similar to that of natural cells, and to maintain stability, they are usually coated with phospholipids or artificial surfactants. Due to the high interfacial tension at the oil-water interface, microdroplets exhibit enhanced stability and maneuverability.
2. Construction of artificial cells on microfluidic chips
1) Preparation of vesicle structures
There are two main classes of methods for constructing liposome vesicles based on amphiphilic molecules.
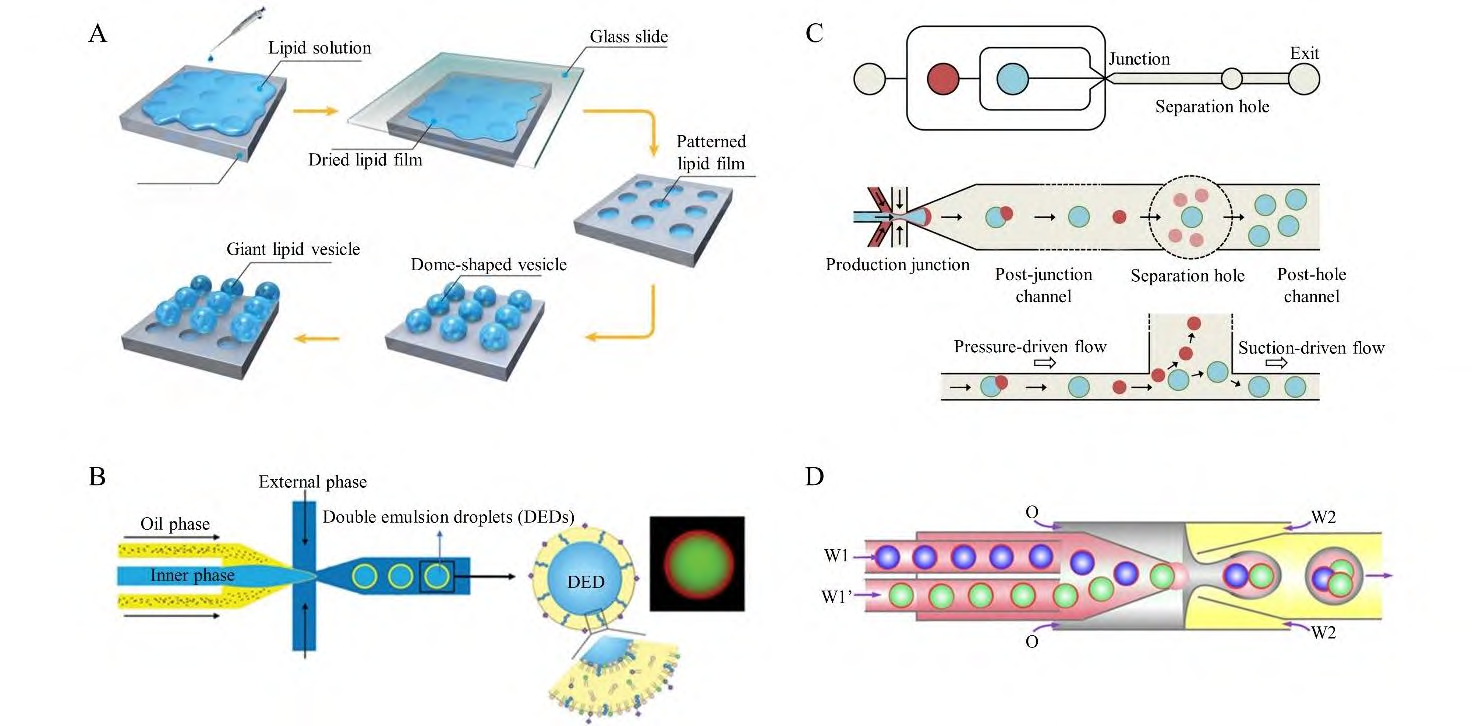
The first type of method is to prepare a lipid bilayer membrane, and then curl it according to a certain law to form closed vesicles to encapsulate the aqueous phase contents. The solid surface is first coated with an organic solvent to form a layered phospholipid dry film, followed by the addition of an aqueous solution to hydrate the phospholipid molecules and generate giant vesicles.
This method is simple and efficient, but the GUVs are not homogeneous in size, the membrane structure is difficult to control, and multiple layers of by-products are generated, and the loading efficiency is low.
The second class of methods is based on emulsion technology, where aqueous-phase droplets generated in the oil phase encapsulate a monolayer lipid membrane through the oil/water interface. By means of phase transfer and other means, the oil phase around the droplet is removed while another lipid film is formed, and finally a bilayer liposome is constructed.
The method starts with the formation of monolayer membrane vesicles with highly uniform structure and size, followed by the construction of bilayer membranes by phase transfer, a process that can be accomplished independently using different microfluidic modules.
Currently, commonly used droplet microfluidic strategies include the water/oil/water (W/O/W) double-emulsion method, in which double-emulsion vesicles are successively generated on a microfluidic chip by encapsulating small droplets with large droplets. The innermost aqueous phase encapsulates the hydrophilic molecules, and the amphiphilic molecules of the liposomes are dissolved in the oil phase, forming a stable W/O/W emulsion before the oil phase is removed.
In addition, techniques such as droplet fission, dielectrophoretic force and electrowetting force have been applied to construct droplet-based artificial cell structures.
With the rapid advancement of micromachining and microfluidics, innovative preparation methods are emerging, such as the use of 3D printing technology to construct artificial cells, which has a high yield and low difficulty, but the vesicles are large in size and not easy to form a complex internal structure.
2) Intracellular compartmentalization construction
Compartmentalization is an important organizing principle in biology that enables complex and well-defined spatial and functional divisions, allows different chemical environments to coexist, establishes chemical gradients, maintains nonequilibrium, and isolates incompatible components.
In order to construct artificial cells that are closer to biological properties, compartmentalized structures are usually formed through self-assembly. Microfluidics enables precise control of the self-assembly process, resulting in the assembly of compartmentalized structures one by one. Multicompartmentalized structures can be constructed by generating multilayered or multivesicular liposomes.
Droplet microfluidics enables effective control of multicompartmental vesicles, precisely defining the number and size of the compartments as well as their lipid composition and internal contents. In addition, inter-compartmental signaling and communication can be achieved through functionalized membrane channels.
Using a multi-step microfluidic approach, a layer-by-layer assembly of nested liposome structures can be assembled to form concentric, eccentric, or multicompartmental liposomes through a dehumidification process with a double emulsion template.
3) Loading of non-membrane components
There are three main ways of loading non-membrane components of artificial cells: the first is the assembly of set components on the membrane and in specific regions within the membrane. The second is to utilize biological or chemical means to generate new components from existing ones, such as using cell-free expression systems to rebuild protein production capacity internally. The third way is to use artificial cellular manipulation means, such as physical perforation, transmembrane transport, etc. to realize the change of components on or within the membrane.
4) Construction of other cellular structures
In addition to membrane structure and loading of contents, it is necessary to construct characteristic structures such as cytoskeleton, cell wall and flagellum. Introducing the cytoskeletal system into artificial cells can change the cell shape and volume through osmoregulation, which in turn can realize complex functions such as migration and mitosis.
Microhydrogel networks mimic the cytoskeleton, enhance liposome resistance to osmotic shock, and are functionalized by immobilized nanoparticles.
The integrity of plant cells is dependent on the cell wall, so it is crucial to mimic the cell wall structure in artificial plant cells. Commonly used materials include cellulose nanofibers and pectin, which mimic the cell wall through the binding of pectin to a network of cellulose chimeric proteins or hemicelluloses. Flagella play a key role in self-propulsion, predation and cellular transport through heterochronic motility.
5) Preparation of Non-Vesicular Artificial Cells
The formation of non-vesicular artificial cells mainly relies on self-assembly, which organizes molecules into ordered structures through multi-scale synergy. Although this method is simple and fast, the generated artificial cells are poorly monodisperse in size and less productive.
Microfluidics can improve the precise control of the self-assembly process. In addition, microfluidic preparation of non-vesicular artificial cells can be achieved by physicochemical polymerization. Droplets can be polymerized into solid particles under heating or UV irradiation.
Site Search
Recommendations
© 2025. All Rights Reserved. 苏ICP备2022036544号-1













