Single cell analysis provides a reliable scientific basis for early diagnosis and treatment of major diseases,drug screening and cell-cell interaction studies. Microfluidic chips, with microchannel structures comparable to cell size and the ability to adjust the size and shape of the channels according to demand, have become a powerful tool for single-cell analysis. Microfluidic chip-based single-cell analysis is characterized by low sample consumption, fast detection speed and high throughput, which is very suitable for the detection of small sample size.
Single-cell capture is a key step in single-cell analysis, and only after successful capture of single cells can subsequent analysis be performed. Microfluidic chips have become the main platform for single-cell capture due to their highly controllable and powerful single-cell processing capabilities.
Microfluidic chip-based single-cell capture methods fall into two main categories:
1. Capture methods based on external forces: single-cell capture is achieved by utilizing external forces such as magnetic, optical, acoustic and electric fields.
2. Hydrodynamic-based capture methods: single cells are captured in microchannels without any external force or labeling, relying solely on fluid flow and inherent differences in cell morphology.The method has virtually no effect on cell activity and is a low-cost, simple and high-throughput method for single-cell capture.
1. hydrodynamic-based microfluidic chip single-cell capture method and principle
Single-cell capture on microfluidic chips based on fluid dynamics is realized by designing microstructures such as obstacles or grooves in the fluid channel and utilizing the interactions between structure and fluid, cell and fluid, and cell and structure by precisely controlling the movement of fluid and single cells in the channel.
Commonly used hydrodynamic single cell capture methods include:Deterministic Lateral Displacement Technique (DLD), inertial microfluidics and filtration technology.
DLD technology utilizes the laminar flow properties of fluids and cell size differences, when cells collide with the micropillar arrays, based on interactions, cells of different sizes flow in different directions. Cells with sizes larger than the hydrodynamic critical size undergo lateral deflection and flow out of the micropillar array with the fluid;
Cells with sizes smaller than the critical size will not be deflected and are intercepted in the gaps between the microposts, thus realizing single-cell capture.DLD technology can separate cells of different sizes and design single-cell capture structures at the exit of the microfluidic chip to realize single-cell capture for different sizes of single cells.
Inertial microfluidics is based on the difference in inertial lift forces on cells of different sizes and the Dean vortex principle for single-cell capture. The size of the cells determines the amount of inertial lift they are subjected to, with larger cells moving towards the inner wall of the channel and smaller cells moving towards the outer wall of the channel.
By designing multiple microfluidic chip outlets, it is possible to realize the separation of different kinds of cells, or to design single cell capture structures at different outlets to realize the capture of multiple single cells. The microchannels for inertial sorting are usually straight-through channels, spiral channels, or combinations thereof.
Filtration technology enables single-cell capture by controlling the microsieve or microwell size based on differences in cell size and deformability. As cells pass through the microsieves or microwells, cells of larger size or less deformability are trapped. By adjusting the size of the microsieve or microwell, a certain single cell can be selectively captured from a mixed cell sample.
2. Hydrodynamic-based single-cell capture structures for microfluidic chips
Depending on the structural design of the microfluidic chip, theSingle-cell capture is mainly categorized into microwell structures, micropillar structures and bypass channel structures.
microwell structure: Design micro-wells that match the size of the single cells, and as the cell suspension flows through, the single cells sink under gravity into each individual micro-well. Since the sinking cells are subject to less fluid impact, they are less likely to be washed away.
microcolumnar structure: Baffles or barriers of various shapes and sizes are designed within the channel to achieve capture by intercepting single cells. Each capture site is designed to hold as many single cells as possible, forming a single-cell capture array.
Bypass Channel Structure: Single cells are captured by differences in fluid resistance at different locations in the channel. As the cell suspension flows along the channel, single cells preferentially enter areas of lower fluid resistance. The captured single cell acts as a “plug”, increasing the fluid resistance at that site and allowing subsequent cells to bypass the occupied capture site and continue to flow to the next capture site.
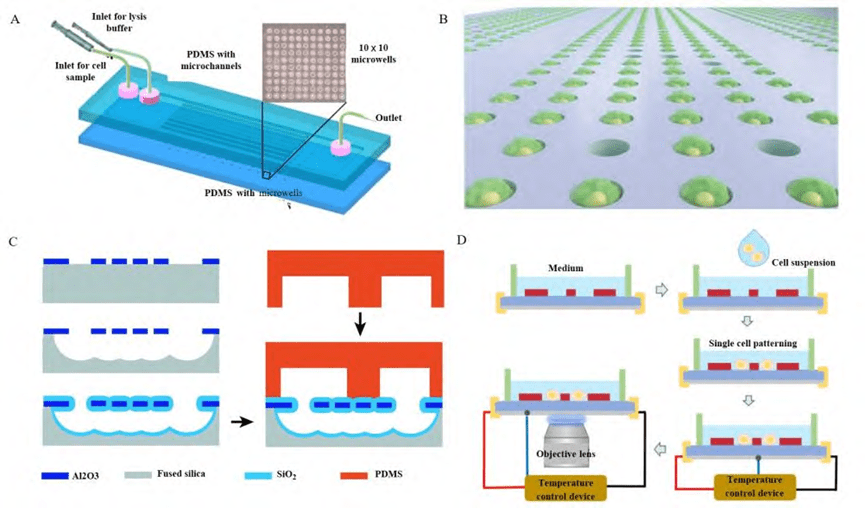
1) Single-cell capture structures based on microwell arrays
Single-cell capture structures based on microwell arrays enable single-cell capture by gravitational sedimentation. This method relies on the matching of single cells to the size of the microwells, allowing cells to be captured in microwells of different sizes and shapes.
As one of the earliest single-cell capture designs, this structure is based on the principle of filtration: the cell suspension is first injected into the microfluidic chip, left for a certain period of time, and the gravity of the cells is utilized to cause them to settle into the microwells, and then the excess cells are flushed away to complete the single-cell capture.
Microwell array-based capture methods can isolate single cells and cell clusters from a large number of cell samples, which are mainly categorized into two types of structures: closed and open.
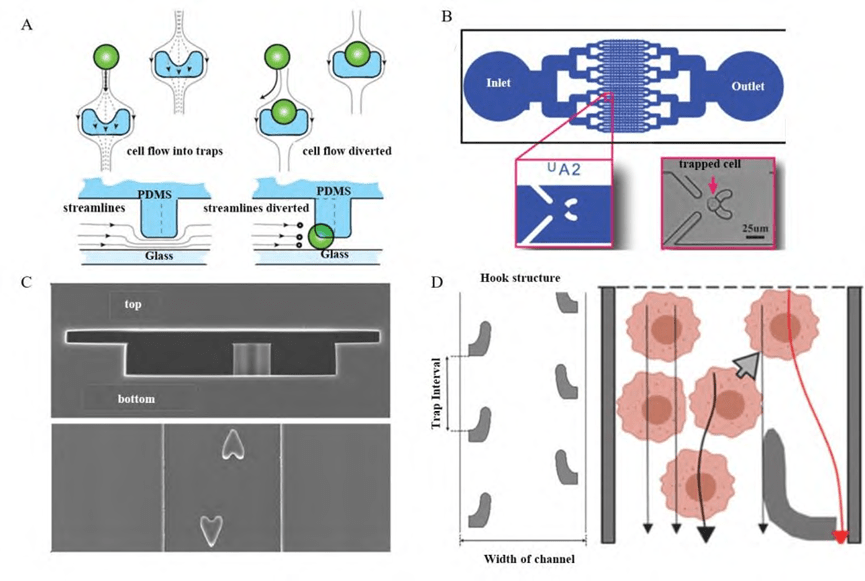
2) Single-cell capture structures based on microcolumn arrays
Micropillar array structures are one of the common designs for single-cell capture, optimizing microbaffles or barriers based on the size of a single cell so that only one cell is captured per micropillar structure.
Through the DLD (deterministic lateral displacement) principle, the micro-pillar array is guided through the fluid flow line to capture a single cell as the cell flows in the fluidic channel. When a single cell is captured, the fluid resistance at that location increases, reducing the probability of capturing other cells. Whether it is a single cell, a cluster of cells, or from whole blood samples to lysed samples, microcolumn structures of all shapes and sizes are effective at capturing them.
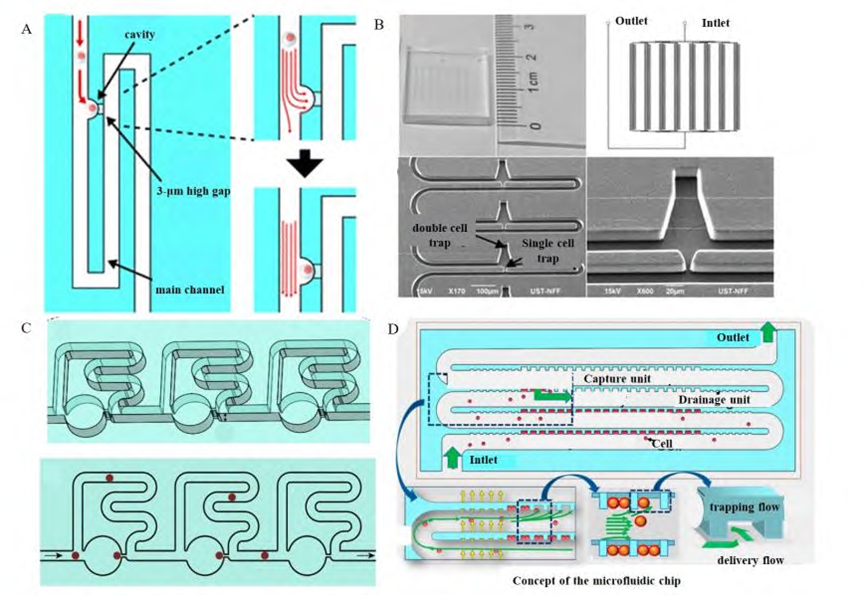
3) Single-cell capture structures based on bypass channels
Single-cell capture structures based on micropillar arrays are usually perpendicular to the main channel flow direction, whereas single-cell capture structures based on bypass channels are parallel to the main channel flow direction and usually consist of a capture site and side channels.
The structure utilizes the principles of DLD (deterministic lateral shift) and inertial microfluidics to design a main channel with higher flow resistance and a side channel with lower flow resistance in the microfluidic chip. When cells flow in the main channel, due to the pressure difference between the main channel and the side channel, the cells will be guided into the side channel with smaller flow resistance. Single-cell capture is realized by a special capture structure at the connection between the main channel and the side channel.
Captured single cells act as “plugs” at the capture site, increasing the fluid resistance at that location and allowing subsequent cells to bypass the occupied capture site and continue on to the next capture site. This design reduces the risk of cell damage by minimizing channel clogging and multicellular aggregation. The single-cell capture structure based on a bypass channel provides a method to sequentially capture single cells and cell clusters while also enabling separation of daughter cells during cell division.
Site Search
Recommendations
© 2025. All Rights Reserved. 苏ICP备2022036544号-1