For the different microfluidic screening devices that have been developed, they are categorized into labeled and unlabeled droplet sorting techniques based on the detection signal.
Labeled Droplet Sorting Technology
Labeled droplet sorting techniques are usually not directly based on the characteristics of the cell or enzyme molecule being assayed, but are indirectly achieved by the introduction of chemical molecules or fluorescent probes.
These techniques mainly include fluorescence-activated droplet sorting (FADS), which relies on fluorescence signals, and absorbance-activated droplet sorting (AADS), which is based on changes in UV/visible absorption.
Fluorescence-activated droplet sorting
FADS is one of the current mainstream microfluidic screening technologies, the core of which is to directly or indirectly couple the target enzyme genotypes with fluorescent signals by designing different fluorescent probes or enzyme cascade reactions, thus providing high sensitivity.
Similar to the FACS principle, the biggest advantage of FADS over FACS is that it can target intracellular, cell surface, secretion to extracellular signals and cell-free systems.
Configuration of a high-speed camera enables real-time visualization of sorting.
Another advantage of FADS over other droplet sorting technologies is its processing throughput of up to 5000 droplets per second.
It has been successfully applied to the modification of enzyme molecules such as lipase, esterase, cellulase, amylase and polymerase.
However, the challenges are the need for customized fluorescent probes and the lack of effective biomarkers and coupling strategies in the face of certain enzyme or small molecule targets, as well as the fact that fluorescent probes have to remain non-spreading within the droplet, limiting their wide application.
In order to overcome the limitations of FADS, other types of droplet sorting techniques have been developed, such as those based on different activation modes such as absorbance, mass and Raman.
Absorbance-activated droplet sorting technique
Absorption spectroscopy is widely used in the fields of colorimetric determination, protein quantification and enzyme kinetics.
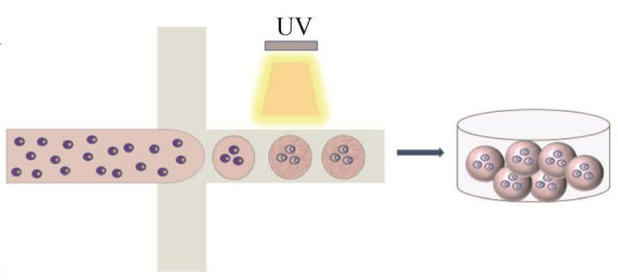
In recent years, researchers have combined absorption spectroscopy with microfluidics to develop absorbance-activated droplet sorting (AADS), which complements fluorescence-activated droplet sorting (FADS).
The challenge of AADS compared to FADS is that the absorbance is proportional to the path length, so the small volume of the droplet (pL) and the short optical path length (μm) affect the sensitivity of the detection.
Theoretically, AADS may have a higher application potential because most small molecules have absorption properties in both the UV and visible regions, making it more universal in comparison.
Label-free droplet sorting technology
Label-free droplet sorting technology utilizes detected reactions, compounds, or physical or chemical changes in the cell itself to achieve signal detection and sorting without the addition of additional chemical molecules.
Label-free sorting methods combining techniques such as mass spectrometry, Raman spectroscopy, nuclear magnetic resonance (NMR), electrochemistry and image analysis have been developed. However, these methods usually require coupling of different techniques and special equipment.
Mass-activated droplet sorting technology
Mass spectrometry (MS) has become a widely used analytical tool as a label-free detection technique.
Conventional liquid chromatography-mass spectrometry (LC/MS) is time-consuming due to chromatographic separations and requires relatively large sample volumes, which makes it cost prohibitive for high-throughput screening (HTS).
In contrast, mass-activated droplet sorting (MADS) combines the advantages of microfluidics and mass spectrometry with high sensitivity, high selectivity, low cost, and simultaneous analysis of multiple products.
Although MS-based droplet sorting methods have been shown to be feasible and more improved methods are gradually being developed, the loss of droplets during processing limits their application in droplet sorting.
Raman-activated droplet sorting
Raman spectroscopy, which utilizes the vibrations and rotations of chemical bonds produced when a laser interacts with a sample to obtain molecular information, has been widely used in the fields of physics, biology, chemistry, industry and medicine.
Raman-activated droplet sorting (RADS) is a label-free, non-invasive single-cell identification and analysis technique that combines single-cell Raman spectroscopy (SCRS) with droplet microfluidics.
The technology captures single cells at high flow speeds and enables Raman spectroscopy acquisition, release and sorting through dielectric single-cell capture and release and solenoid valve sucking techniques.
However, the main drawbacks of this technique are the susceptibility to optical aberrations in the oil phase and in the droplets, the low sensitivity when surface enhancement is not used, and therefore the limited application when dealing with complex matrices.
Theoretically, RADS is applicable to all types of cells and has a wide range of applications.
However, current applications based on Raman spectroscopy still require high Raman intensities and large cell scattering cross sections. Additional optimization is needed when applied to small cells such as E. coli.
Nuclear magnetic resonance-activated droplet sorting technique
Nuclear Magnetic Resonance-Activated Droplet Sorting (NMR-ADS) is not only a label-free sorting technique, but also a non-destructive sorting method compared to MADS.NMR is capable of identifying and quantifying hundreds of compounds in complex mixtures simultaneously, with unparalleled chemical specificity.
Combining NMR with microfluidics is immensely attractive, but it also presents major challenges. A challenging class of key components from the NMR point of view is the conducting structures.
Metal electrodes, while available for electrochemical sample interactions, can lead to significant degradation of NMR spectra and signal-to-noise ratio (SNR). These problems are compounded at the microscopic scale, where the distortion volume is a much larger proportion of the sample.
In addition, the detection sensitivity of NMR is low (less than 1 mmol/L) compared to other detection methods. NMR microcoils have been developed to address these limitations, but the challenge of interfacing microcoils with small samples remains.
Electrochemically activated droplet sorting
Electrochemical detection is likewise a label-free, non-destructive assay suitable for the analysis of complex samples.
However, due to the limitations of the electrode surface size (which must be smaller than the droplet size), the droplet size can usually only be controlled at the nanoscale level.
Droplet sorting technique based on image analysis
Image analysis-based droplet sorting (IBDS) is a label-free sorting technique that works by identifying, processing, and analyzing the morphological features of the cells within a droplet.
In recent years, the method has become increasingly popular in the field of cell biology, due in part to improvements in image processing algorithms, interface speeds, and advances in processing hardware performance.
About Us
DingXu (Suzhou) Microfluidics Technology Co., Ltd. is a high-tech enterprise dedicated to the field of microfluidics. We are committed to providing customers with comprehensive microfluidic solutions, including customized microfluidic chip development, surface modification, microfluidic chip processing equipment, and microfluidic instruments. Our team boasts extensive experience and technical expertise, continuously combining professional knowledge with innovative thinking to deliver high-quality solutions. We consistently prioritize customer-centric values, embrace self-challenges, and pursue excellence. Through professionalism, innovation, and collaboration, we aim to create greater value for our customers and contribute to a brighter future in the field of microfluidics.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1