cancer
Cancer is one of the most dangerous diseases to human health in the 21st century, and roughly 10 million people worldwide die of cancer every year.
However, there is still a lot of room for improvement in the current medical technology tools. The first CellSearch system approved by the U.S. Food and Drug Administration (FDA) has already been applied in the clinical CTC detection system, which can be used for efficacy monitoring and prognostic evaluation of a variety of cancers, and can provide information on the prognosis of patients with metastatic colorectal, breast and prostate cancers.
However, the platform's reliance on epithelial markers and exclusion of immune antigen-expressing cells failed to capture important subpopulations with prognostic value.
And because of the CellSearch system's low sensitivity, it is almost exclusively used for late-stage cancer detection, so it cannot effectively detect early and mid-stage cancer progression.
But especially for patients in the early stages of cancer, it is the best time to treat cancer if it is detected in time and therapeutic interventions are taken.
CTC
In recent years, scientists have discovered a new testing technique, liquid biopsy.
Liquid biopsy is a minimally invasive method for detecting tumor-derived markers in body fluids with prognostic or diagnostic utility for early diagnosis, metastasis tracking, drug resistance prediction, and prognostic recurrence monitoring.
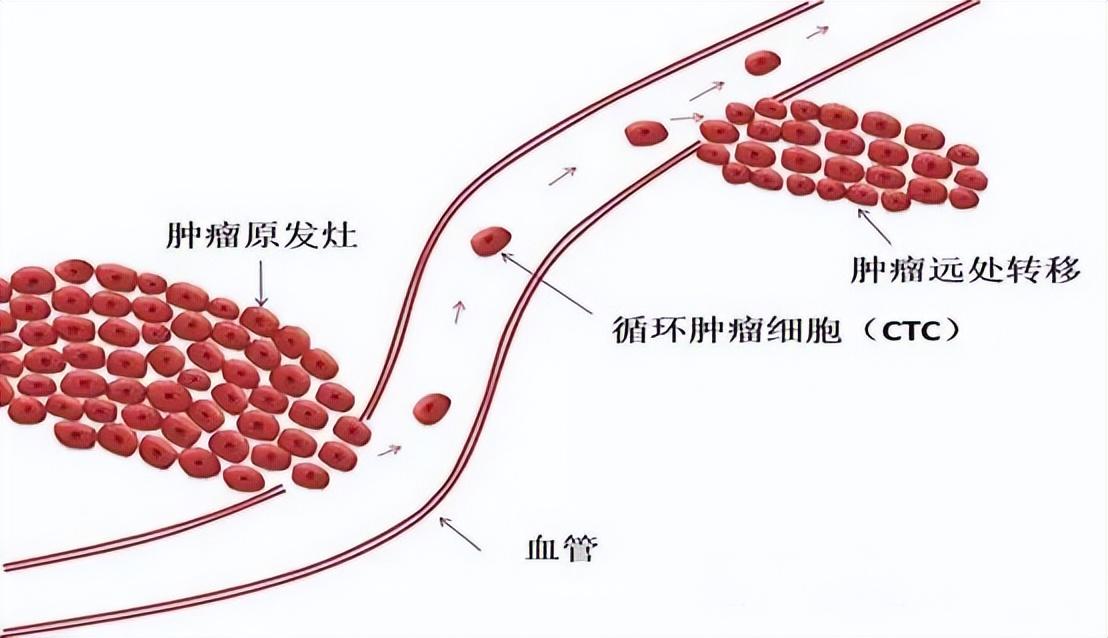
As one of the important markers for liquid biopsy, the level of circulating tumor cells (CTC) in the peripheral blood can be used to assist in determining a patient's cancer disease status.
In addition to this, CTC is also very important for basic research such as the metastatic behavior of tumor cells.
However, the content of CTC in human blood is extremely scarce, usually only 0-10/mL, in contrast to the content of red blood cells, white blood cells and platelets, which reaches 5×109/mL, 4×106/mL and 3×108/mL, respectively, and the tumor cells in the metastatic process can be transformed by epithelial⁃mesenchymal transition (EMT) and mesenchymal⁃epithelial transition (MET) to continuously change their own characteristics.
It is due to its scarcity and heterogeneity, as well as the interference of complex matrices in the blood, that accurate detection of CTCs becomes a huge challenge.
Routine testing techniques
Since conventional optical analytical means are difficult to meet the requirements for direct detection in terms of both detection limit and sensitivity, the separation and enrichment of CTC in peripheral blood are usually achieved by some sample pretreatment methods prior to the detection of CTC in peripheral blood.
Frequently used sample pretreatment methods can be categorized into physical and chemical methods.
Physical methods are mainly based on the differences in physical characteristics of the cells to be separated, such as membrane filtration separation and density gradient centrifugation, is based on the size of the cells and the density of the cells to complete the screening, respectively.
Chemical methods, on the other hand, rely mainly on the specific recognition of biological macromolecules, such as antigen-antibody interactions and selective binding of nucleic acid aptamers to targets.
Conventional detection of technical deficiencies
Although the above sample pretreatment methods can realize the separation and enrichment of CTC to varying degrees, they also have certain defects.
Since these methods are discontinuous, the process of adsorption, elution and transfer inevitably results in the loss of cells, which, together with the scarcity of CTC itself, can easily lead to the generation of false-negative results.
Assays incorporating microfluidic chips
This problem can be well solved by utilizing the integrated function of microfluidic chip. The operations of CTC capture, release, counting and detection can be completed on the chip, and the continuous automated processing can effectively reduce the interference of human error.
In addition, the microfluidic chip requires a very small injection volume, which can greatly reduce the consumption of precious samples and reagents and lower the cost of testing.
And the effect of surface force will be obviously amplified under the micro-scale, which can effectively improve the efficiency of substance mixing and reaction, and realize rapid and efficient separation and analysis.
Therefore, several studies in recent years have attempted to utilize the microfluidic chip platform to carry out CTC separation and detection with good results.
Physical characterization-based approach to microfluidic chips
a) Size and deformability
Screening for CTCs based on differences in cell size is one of the isolation methods that have been used for a long time, and this technical approach is relatively simple and inexpensive.
A microfluidic chip that enriches CTCs based on their size and deformability and automatically stains them for cell identification and subsequently recovers cells from this microfluidic chip outlet has been reported.
Using this Microfluidic Chip, the average cell capture efficiency was 42% to 70%. Most importantly, CTCs recovered from patient samples processed using this Microfluidic Chip have a high survival rate and can still be used for downstream molecular analysis.
b) Fluid dynamics
According to the literature, the isolation of circulating tumor cells CTC by microfluidics is a relatively efficient method, which mainly combines microfluidics with fluid dynamics.
When the fluid flows in the microchannel, the suspended cells in the fluid collide with the interior of the microfluidic chip, generating a certain force, and cells of different sizes will generate a different flow trajectory, so that the circulating tumor cells CTC can be separated from the blood cells.
Deterministic lateral displacement chip (DLD)-based separation is a technological approach to separate CTCs from blood cells based on a combination of cell size and fluid dynamics.
According to the principle of DLD, large particles (e.g., cancer cells) exceeding the design critical size will flow along the inclined axis of the array, while fluids and small particles will flow in the horizontal direction of the array.
Using this microfluidic chip it is possible to isolate more than 85% of CTCs from a few milliliters of clinical blood samples in a matter of minutes and without compromising cell viability for the next step of culturing and analyzing the cells.
c) dielectrophoresis
Different cells have different dielectric parameters because of their different properties such as diameter, membrane capacitance, cytoplasmic conductivity and other different physical properties.
Dielectrophoresis is a method used to separate cells from other molecules or cells from each other. Cells with different properties produce different dielectric forces under the same electric field, and since circulating tumor cell CTCs are not the same as blood cells, the dielectrophoresis method can be used for the separation of CTCs from blood cells.
A related study has designed a microfluidic chip with circular microelectrodes that generate a stepped electric field by switching the electric field between neighboring electrode pairs via a relay, which can effectively isolate cervical cancer cells (HeLa) from peripheral blood samples.
A microfluidic chip approach based on chemical characterization
a) Antibody Recognition
Among the technical methods for isolating CTCs from circulating tumor cells, antibody affinity-based isolation methods are one of the most common methods for CTC isolation.
Epithelial cell adhesion molecule (EpCAM) plays a role in the process of epithelial cell carcinogenesis and is an important biomarker for distinguishing CTCs from blood cells.
By modifying the EpCAM antibody into the internal structure of the microfluidic chip, when the sample is passed into the microfluidic chip, the EpCAM antibody will specifically recognize the target CTC and form a non-covalent bond with the target, and then the CTC will be captured in the microfluidic chip, while the other cells will flow out with the buffer.
A related report describes a new microfluidic chip (SDI-Chip) for efficient and highly specific CTC capture and detection.
b) Nucleic acid aptamer recognition
Nucleic acid aptamers are small segments of single-stranded DNA or RNA that have been screened in vitro to form non-covalent bonds and bind specifically to a target ligand.
Nucleic acid aptamers bind to their targets mainly by helical folding to complementary sequences of the target ligand, whereas antibodies bind to their targets mainly to antigenic clusters specific to the surface of the target antigen.
One study points to a microfluidic chip that uses a sub-10-nanometer three-dimensional DNA structure as a framework with an aptamer modified on top.
The vertical nanoframe ensures that the aptamers are in a highly ordered upright orientation, which is more conducive to the contact of the aptamers on the microfluidic chip with the CTCs in the samples and improves the capture efficiency of the CTCs.
Other Expansions - 3D Printed Microfluidic Chips
Traditional microfluidic devices are based on photolithographic manufacturing processes, where complex manufacturing steps, expensive material costs, and time-consuming fabrication processes all limit the translation of microfluidic devices from the laboratory to actual clinical implementation.
In contrast, 3D printing technology offers relative flexibility, immediacy, and the ability to rapidly prototype a wide range of complex three-dimensional structures layer-by-layer, increasing the functional surface area of microfluidic chips and facilitating precise and small-scale microfluidic chip fabrication.
In addition, the availability of a variety of suitable raw materials will continue to enrich the range of applications for microfluidic devices and promote their widespread acceptance in both research and clinical settings.
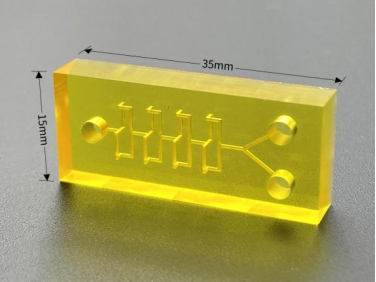
3D printed microfluidic chips could open up new opportunities for CTC detection and early monitoring of tumor metastasis, and although there are currently few research applications for such chips, it can be predicted that 3D printing technology will become one of the most important methods in the field of microfluidic chip fabrication in the future, providing useful insights for the widespread clinical application of CTC detection.
About Us
DingXu (Suzhou) Microfluidics Technology Co., Ltd. is a high-tech enterprise dedicated to the field of microfluidics. We are committed to providing customers with comprehensive microfluidic solutions, including customized microfluidic chip development, surface modification, microfluidic chip processing equipment, and microfluidic instruments. Our team boasts extensive experience and technical expertise, continuously combining professional knowledge with innovative thinking to deliver high-quality solutions. We consistently prioritize customer-centric values, embrace self-challenges, and pursue excellence. Through professionalism, innovation, and collaboration, we aim to create greater value for our customers and contribute to a brighter future in the field of microfluidics.
Site Search
Recommendations
© 2025. All Rights Reserved. 苏ICP备2022036544号-1