Micromachining technology offers great business opportunities for biomedical instruments. Needles are one of the most common and simple biomedical instruments. Typically, hypodermic needles are used in the human skin to deliver drugs and aspirate body fluids.
The smallest diameter of needles manufactured through conventional processing methods is approximately 300 micrometers. These relatively large needles cause pain and are less accurate for targeted drug delivery at the micrometer scale. In addition, advances in biotechnology require delivery of nanoscale molecules with micrometer precision. Conventional hypodermic needles cannot meet these requirements. Translated with DeepL.com (free version)
Current micromachining technology has made it possible to manufacture needles smaller than the 300 micron limit size. Microsize technology has opened up new areas of application for this simple device. The main applications of microneedles include:
Painless administration and injection of vaccines through the skin (transdermal or intradermal);
Minimally invasive ocular drug delivery;
Provides active cosmetic ingredients;
Patient monitoring and diagnosis;
Closed-loop chemical stimulation of tissues;
Cell manipulation;
Sample collection and transport in chemical and biochemical analysis.
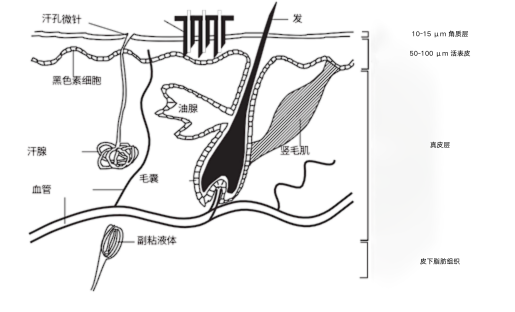
The figure below shows a schematic of the structure of human skin. The outer layer is the stratum corneum (SC), which is 10 to 15 microns thick and is a dead tissue. The stratum corneum provides protection for the body.
However, this layer also constitutes a barrier that limits the rate of drug transfer across the skin during transdermal administration. The next layer is the living epidermis (VE), which is about 50 to 100 micrometers thick. This layer of tissue consists of living cells with blood vessels capable of transmitting drugs, but few nerves.
Things like needle injections, chemical/lipid enhancers, iontophoresis, electroporation, acoustic and photoacoustic effects can increase transdermal transfer rates, and direct injections can deliver drugs subcutaneously.
While other methods create small holes in the surface layer of the skin to increase the rate of drug molecule delivery. Most of these methods create holes on the submicron scale.
Microneedles (MN) are an alternative to delivery channels, with pore sizes of approximately micrometers, large enough to achieve high delivery rates but still incapable of causing clinically significant damage. In addition, microneedles allow for highly localized and even intracellular drug delivery.
If the microneedle can reach the VE area, the drug can be administered painlessly. The microneedles should penetrate the skin 15 to 100 micrometers to achieve this. An array of microneedles is required to penetrate the skin during transdermal drug delivery.
Microneedle holes create microscopic pores that accelerate drug diffusion. Microneedle arrays are considered to be minimally invasive drug delivery devices that bypass the skin barrier to deliver drugs to the skin microcirculation. This represents systemic drug delivery via the transdermal route.
Microneedles can also remain in the skin after insertion. An array of solid microneedles entrapped with a drug gel may increase the rate of delivery through the gap between the needle and the punctured skin. In addition to the puncture function, the microneedles may also have a delivery function.
Drug-coated microneedles can achieve higher delivery rates. Hollow microneedles can deliver drug directly intradermally to the VE area. The drug is delivered through the holes inside the microneedle by diffusion or pressure-driven flow.
Since micromachining technology is compatible with microelectronics, more functions can be integrated in the needle. Monolithic integration of sensors in microneedles can measure tissue and cellular responses.
The chemical stimulatory function of the drug and the response signal of the sensor make it possible to control cell stimulation in a closed loop. Microneedles can manipulate individual cells and measure their responses. Another application for microneedles is precise liquid sampling for chemical and biochemical analysis. The small size of microneedles increases the resolution of the dispensed volume.
Microneedles can be categorized into in-plane and out-of-plane needles based on the orientation of the insertion direction relative to the substrate surface. Microneedles can be categorized into solid and hollow needles based on their tip. Each type can be further categorized according to its substrate material (e.g., silicon, metal, polymer, or glass).
Administration by the transdermal route has the following advantages:
Inhibits degradation in the gastrointestinal tract;
Prevents first pass hepatic metabolism;
Using the same patch, relatively stable drug concentrations in plasma can be maintained for up to 7 days;
Avoid pain, discomfort and poor compliance caused by injections.
In order to utilize MN arrays for drug delivery, efforts have been made to explore a variety of materials, fabrication methods, and designs to develop MN technology for drug delivery applications.MNs are fabricated in heights ranging from 50 μm to 900 μm and in densities up to 2,000 MN/cm2.
According to available information, MN can be classified into five types: solid, coated, hollow, dissolvable and hydrogel molding.
Solid MN requires a two-step delivery method. First, temporary microwells are created in the SC by MN. Then the drug formulation is used. The drug is transported by passive diffusion. The role of solid MN is to enhance the penetration of the drug formulation. Silicon, metals and polymers have been used to manufacture solid MN.
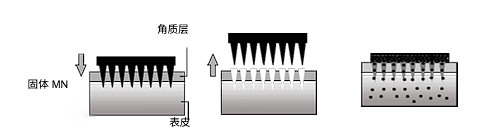
The second method is the use of drug-coated MNs. prior to dermal application, the drug formulation is coated onto solid MNs. This is a one-step drug application process, but the amount of drug applied is limited by the limited surface area of the MN array. Coated MNs have been used for rapid dermal delivery of vaccines, proteins and peptides.

Dissolvable MNs are made from biocompatible polymers or sugars by a micro-molding process.The MNs contain pharmaceutical agents. After skin insertion into the MN array, the MN dissolves and is subsequently exposed to the skin mesenchyme.
Controlled release of drugs can be achieved by adjusting the fabrication process of MN or regulating the polymer composition of MN. Water-soluble materials or biodegradable polymers have been used to fabricate MN arrays. Upon insertion into the skin, the MN dissolves or degrades to release the drug.

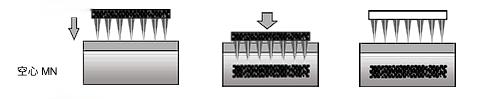
Hollow microneedles allow liquid preparations to be injected directly into the skin by means of a micromechanical device inside the needle. Drug injection can be accomplished using various actuation methods such as diffusion, pressure or electricity. This drug delivery scheme is capable of injecting more drug than other methods. However, drug injection may be limited due to clogged needle holes.
To solve this problem, the design of the MN can be modified by creating a hole on the tip side of the MN needle. Special attention should be paid to designing a suitable reservoir to hold the liquid drug under safe and stable conditions. Liquid drugs can be very unstable, especially at high temperatures.
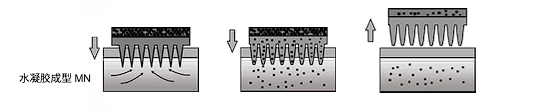
The last method is a relatively new method that uses a hydrogel to form the matrix.MN is prepared from cross-linked polymers and does not contain the drug. The drug is loaded into a patch reservoir located above the array. After insertion of the MN array into the skin, tissue interstitial fluid diffuses into the polymer MN, allowing the drug to be transferred from the reservoir to the tissue via a swollen needle.
Hydrogel-forming needles can be withdrawn from the skin intact, but MN arrays cannot be reused because of the softened tip. This feature reduces the risk of infection transmission.
The requirements for microneedle tips depend on their application. The general requirements for microneedles are as follows:
Sharpness of penetrating tissue;
Resistance to fracture, bending and flexure strength;
Sufficient flow rate in the pinhole;
Biocompatibility of needle materials;
Operational considerations for clinical translation.
The use of MN should not lead to any infection as MN penetrates the saprophytic layer of the skin. MN sterilization or sterile MN production should be performed to inhibit the transmission of any microbial load to the body and to reduce the risk of infection. The design of the required processes should not compromise the design of the MN and its cargo. In addition, the biocompatibility of the materials used in the manufacture of MN is necessary to avoid local or systemic reactions.
More importantly, the design of MN-based drug delivery should be optimized to improve patient and prescriber compliance.MN patches should provide easy-to-use alternatives to oral and parenteral drug delivery. To this end, an applicator (e.g., a spring-loaded piston) may be used to insert the MN to minimize individual variation during dermal insertion. For administration, the applicator is placed on the surface of the skin and pressed to allow the MN to penetrate the epidermis.
About Us
DingXu (Suzhou) Microfluidics Technology Co., Ltd. is a high-tech enterprise dedicated to the field of microfluidics. We are committed to providing customers with comprehensive microfluidic solutions, including customized microfluidic chip development, surface modification, microfluidic chip processing equipment, and microfluidic instruments. Our team boasts extensive experience and technical expertise, continuously combining professional knowledge with innovative thinking to deliver high-quality solutions. We consistently prioritize customer-centric values, embrace self-challenges, and pursue excellence. Through professionalism, innovation, and collaboration, we aim to create greater value for our customers and contribute to a brighter future in the field of microfluidics.
Site Search
Recommendations
© 2024. All Rights Reserved. 苏ICP备2022036544号-1