3D cell culture is the cultivation of cells in a three-dimensional environment that mimics the structure of tissues and organs, enabling the cells to grow in three dimensions and interact with their surroundings.
The advent of this technology remedies the lack of cell-extracellular matrix (ECM) interaction in 2D culture.
1. 3D cell culture models
One of the key foundations of 3D cell culture technology is the extracellular matrix (ECM). the ECM consists of a variety of extracellularly secreted proteins (e.g., collagen, elastin, laminin, and glycosaminoglycans), as well as cell-binding factors (e.g., CD44, and integrins, etc.), which form an intricate network structure.
ECM not only provides important structural support for cells, but also participates in the regulation of cell growth, migration, differentiation, survival and homeostasis, and directs tissue morphogenesis.
By reconstructing the ECM in vitro, 3D cell culture is able to mimic the in vivo environment and promote cells to form three-dimensional structures similar to in vivo tissues.
This technology allows cells to grow in more complex morphologies, so gene expression is closer to the in vivo state and is tissue-specific, providing a truer picture of the physiology of in vivo tissues and providing in vivo-relevant data.
Compared with 2D culture, 3D culture showed significant differences in cell morphology, migration and gene expression.
Mesenchymal stem cells (MSCs), a class of somatic cells derived from tissues such as bone marrow, umbilical cord, or adipose, have great cellular therapeutic potential, but due to their limited number of divisions, they eventually undergo replicative senescence, leading to apoptosis.
2. Types of 3D culture models and construction methods
1) 3D culture model with scaffolding
Scaffold-based 3D cell culture utilizes natural or synthetic polymers to mimic the environment of the extracellular matrix (ECM) in vivo, thereby promoting cell growth and proliferation.
These scaffolds need to have specific chemical composition, porosity, and mechanical properties to accommodate cellular heterogeneity and facilitate cell-cell and cell-ECM interactions, contributing to cell proliferation, migration, differentiation, and adhesion, ultimately leading to the formation of spheroids or organoids in vitro that resemble in vivo structures and functions.
Natural scaffold materials, including alginate, agar, gelatin, collagen, and sericin proteins, are biocompatible with cells and can regulate cell behavior by providing a similar matrix environment in vivo and secreting growth factors and signaling molecules.
However, the limitations of natural materials are also evident, such as batch-to-batch variation, varying purity, poor reproducibility, and sterilization difficulties.
To address these issues, researchers have developed synthetic polymers as scaffolding materials including pyrolyzed carbon, polyvinyl alcohol, polycaprolactone, and polyethylene glycol, which are more controllable and allow precise adjustment of their parameters, but usually lack the biocompatibility of natural materials.
Currently, many 3D culture systems use composite scaffolds of natural and synthetic materials.
Synthetic polymers modulate cell adhesion and enhance cell spreading, proliferation, differentiation, and migration, thereby compensating for the poor controllability of natural materials and improving the biocompatibility of synthetic materials.
2) 3D culture model without stent
Scaffold-free 3D cell culture technology relies heavily on the self-aggregating properties of cells, which are induced to form spherical aggregates through specific culture conditions.
These conditions include, for example, the use of low-adhesion materials or ultra-low-adhesion (ULA) culture plates that allow cells to aggregate with each other and form three-dimensional structures.
The size of the cell spheres can usually be controlled by adjusting the amount of cells inoculated and the droplet volume.
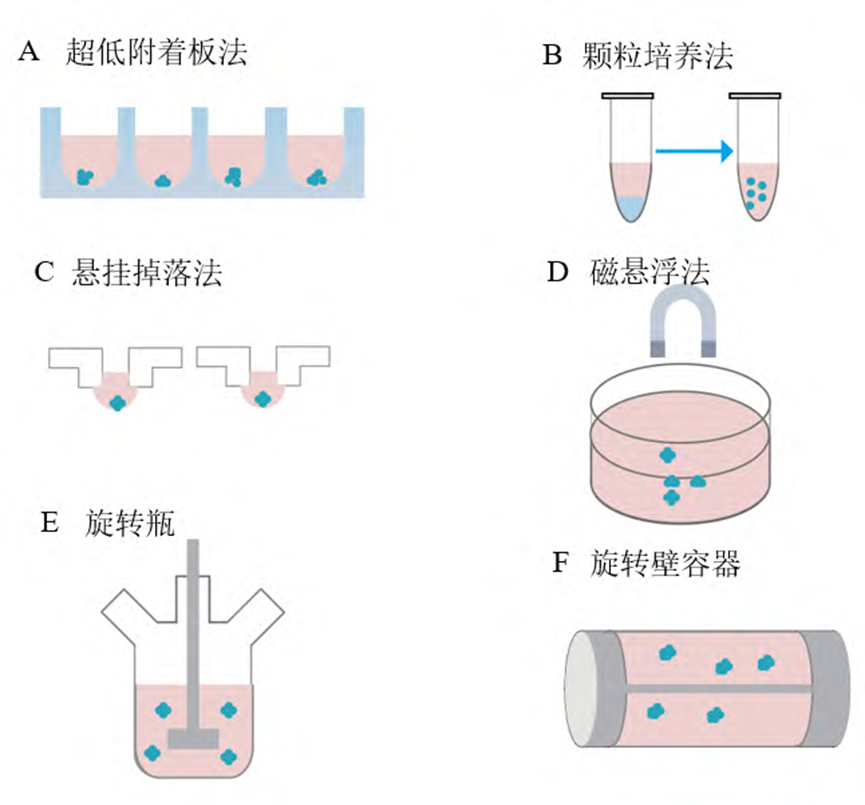
Scaffold-free culture methods include ultra-low attachment plates, pellet culture methods, suspension drop methods, magnetic levitation methods, and bioreactors, each of which produces 3D cell cultures of different morphologies.
Pellet culture is a simple 3D culture technique in which cell suspensions are concentrated by centrifugation to form pellet spheres, and these pellets are suspended in the culture medium, which helps to increase intercellular adhesion and thus promotes the formation of spheroplasts.
The hanging drop method involves making a suspension of 2D-cultured cells, inoculating them into a droplet in a mini tray, and then inverting the tray so that the cells self-aggregate in the droplet to form a 3D sphere with the help of gravity and surface tension.
This method can be used to control the size of the spheres by adjusting the droplet size and the concentration of the cell suspension.
By mixing cells with magnetic nanoparticles, the magnetic force, gravity and buoyancy are balanced under the action of an external magnetic field, which suspends the cells in the culture medium and promotes the assembly and interaction of the cells, ultimately leading to the formation of cell aggregates.
Bioreactor technology includes rotating vials and rotating wall vessel systems.
Spinner bottles are rotated by a motor-driven impeller to mix cells with the culture medium and prevent cell deposition, but the fluid shear generated by the system may have an effect on the cells, so precise regulation of the rotational speed is required.
Rotating wall containers, on the other hand, generate microgravity and low shear forces by rotating, in which cells are suspended and aggregated into spheres.
The scaffold-free culture system mainly utilizes the aggregation of cells and inhibits the attachment of cells to the culture plate in different ways so that the cells can self-aggregate and form three-dimensional structures.
In contrast, 3D culture systems with scaffolds mimic the in vivo ECM environment by providing structural support and facilitating cell-matrix interactions to form more complex organoids.
There is no fixed procedure for 3D cell culture, and there are a variety of technical approaches.
In addition to scaffolding or self-aggregating properties, 3D cell culture has been combined with microfluidics to form microfluidic chips or bioprinting technology, demonstrating great potential for life science research.
Scaffold-less and scaffolded culture systems have their own advantages and limitations: scaffold-less systems are easy to manipulate and are suitable for simple spheroid cultures, whereas scaffolded systems are capable of culturing more complex 3D cellular models that mimic the ECM environment in vivo and support tissue generation and organoid formation.
3. Application of 3D cell culture technology
It has been shown that tumor-like models constructed using 3D culture technology can recapitulate key features of tumors, such as the tumor microenvironment, cell-to-cell, and cell-ECM interactions, which provides an important platform for in-depth study of the mechanisms of cancer onset, progression, and drug-resistant recurrence.
Currently, 3D cell culture technology has been successfully applied to the modeling of breast cancer, colon cancer, skin cancer, macrophages and other related tumor cells.
These models are important for drug development, therapeutic efficacy assessment, development of personalized treatment protocols, and immunotherapy research.
Stem cells have strong differentiation potential, and researchers have successfully cultivated stem cells through 3D cell culture technology to construct models of brain-like organs, fat-like organs, bone-like organs, and hair follicle-like organs, which are used to study disease mechanisms, tissue development, and as an alternative source of cells and tissues for transplantation.
Extracellular vesicles are lipid bilayer particles produced by stem cells through the paracrine pathway and are an alternative to stem cell therapy.
Culturing stem cells using 3D cell culture technology not only improves the production and activity of extracellular vesicles, but also increases their content, which enhances the therapeutic effect.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1













