1. Conventional cell separation and concentration techniques
Conventional cell separation and concentration techniques rely heavily on the physical properties of cells, such as density, size and shape. These techniques typically utilize physical principles such as centrifugation, filtration, or sedimentation to concentrate cells from a dilute sample to a smaller volume for subsequent analysis or processing.
Common conventional cell separation and concentration techniques can be categorized as follows: centrifugation, ultrafiltration, precipitation and immunoaffinity capture.
1) Centrifugation
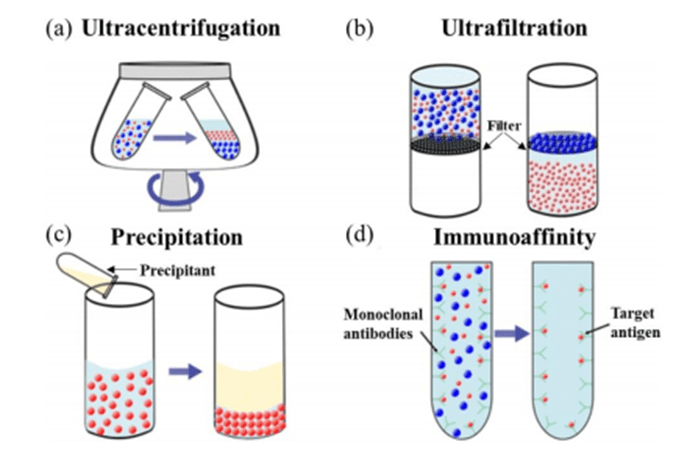
Centrifugal methods utilize differences in size, shape and density of biological particles to achieve separation, relying on centrifugal force generated by ultra-high rotational speeds to deposit the particles. The main categories are density gradient centrifugation and differential centrifugation.
Density gradient centrifugation stratifies and concentrates particles by creating a continuous or discontinuous density gradient in the centrifuge tube, causing the particles to settle according to the density difference. Differential centrifugation concentrates particles at the bottom of the tube by controlling the centrifugation speed and time according to the settling velocity of different particles.
Although centrifugation is widely used and considered the gold standard for particle separation, it has low throughput, long processing times, high equipment costs and requires specialized skills to operate. In addition, high rotational speeds can lead to damage to biological particles, affecting subsequent analysis.
2) Ultrafiltration
Ultrafiltration separates particles by means of a membrane with a specific pore size structure. Ultrafiltration membranes with different pore sizes are selected according to the requirements to allow the penetration of particles of different sizes.
By applying pressure or centrifugal force, particles and solvents will penetrate the membrane and form a leachate.
The ultrafiltration method is simple to operate, has low energy consumption, and compact equipment, but it has some drawbacks, such as the pore size is prone to deformation and clogging, which leads to a decrease in particle flux and recovery. In addition, the pressure to which the particles are subjected while passing through the ultrafiltration membrane may impair their morphology and function.
3) Sedimentation
Sedimentation achieves particle separation by adding specific chemicals (coagulants or flocculants) to the suspension.
These chemicals interact with particles and cell surface components, causing them to aggregate into larger clumps or flocs that can be easily separated by sedimentation or filtration.
Although the precipitation method is commonly used for cell or particle concentration, its limitation is that the choice and dosage of coagulant need to be strictly controlled, otherwise it may have adverse effects on the particles.
4) Immunoaffinity capture method
Immunoaffinity capture methods utilize specific interactions between antibodies and antigens to separate biological particles from particle suspensions and are widely used in fields such as biochemistry and molecular biology.
Many biological particles contain antigens on their surfaces that can bind to specific antibodies, thereby immobilizing the antibody or antigen on the solid phase surface to form an immunoaffinity column.
Unbound non-specific particles are then freely dispersed in the culture medium and can be eluted, accomplishing the isolation and concentration of the target bioparticles. However, the immunoaffinity capture method still faces the challenges of low yield, limited availability of specific antibodies, and possible damage to biological particles.
2. Active microfluidic manipulation technology
Active microfluidic manipulation technology controls the flow of biological particles such as particles, cells and bacteria in the fluid by applying an external field to generate an additional force, creating a stable focused flow and separating the target sample from the waste stream, reducing the volume of the target sample for separation and concentration.
Depending on the applied external field, active microfluidic manipulation techniques can be mainly classified into the following categories: magnetic field manipulation, acoustic field manipulation and electric field manipulation.
1) Magnetic field manipulation
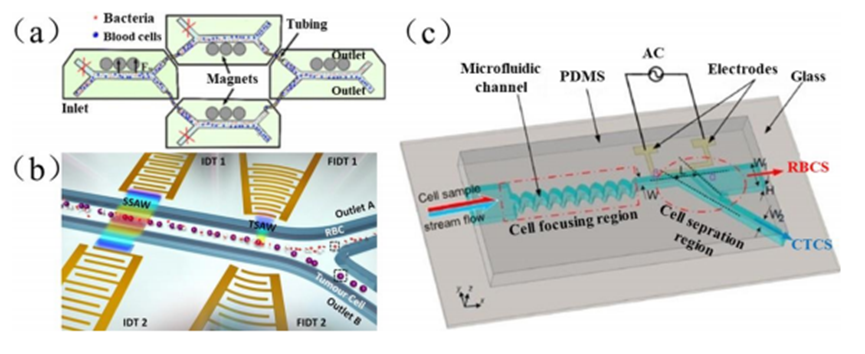
The technique utilizes an external magnetic field to control the movement of particles in the fluid. Separation is achieved by generating a magnetic force in the microfluidic channel by means of a permanent magnet or electromagnet to manipulate magnetically responsive red blood cells or other magnetic target particles to migrate within the microfluidic channel and form a focused flow.
Magnetic field manipulation microfluidics has the advantages of low cost and simple operation. However, the sample preparation process is more time-consuming and laborious, and the separation efficiency depends on the loading capacity of the magnetic beads. In addition, the accumulation of magnetic nanoparticles and prolonged exposure to magnetic media may affect the integrity of biological particles.
2) Sound Field Manipulation
The technique utilizes acoustic waves to drive the movement of particles, specifically by applying acoustic radiation force through ultrasonic standing waves, causing the particles to migrate within the microfluidic channel and exit through a specific outlet for focusing, sorting, and separation.
The intensity of the acoustic radiation force is affected by particle size, density and compressibility. This technique has the advantages of being non-contact, biocompatible and versatile, but its low processing throughput, complex manufacturing process and the need to select suitable materials to effectively apply ultrasonic standing waves limit practical applications.
3) Electric field manipulation
The technique utilizes the effects of electrophoresis, dielectrophoresis and electroosmosis to control the movement of particles in the fluid by applying an electric field, and its mediated electrophoretic microfluidics is the most widely used in this field.
Dielectrophoresis (DEP) is a technique in which when a non-uniform electric field is applied to particles with dielectric properties, the particles are subjected to dielectrophoretic forces and migrate along the electric field gradient according to their size, shape, and dielectric properties to complete the separation.
The technique is simple, easy to operate and low cost, but the electric field may affect the activity of the biological particles, while the adhesion and chemical reaction of the biological particles on the surface of the electrodes may lead to a decrease in the recovery rate.
3. Passive microfluidic manipulation
Passive microfluidic manipulation technology does not rely on the force of an external field, but rather utilizes hydrodynamic forces, the geometry of the microfluidic channel, or special fluidic effects induced by this structure to achieve precise manipulation for focused separation based on the differences in size, shape, and density of biological particles.
Common passive microfluidic manipulation techniques include micro-barrier filtration, deterministic lateral displacement (DLD), and inertial microfluidics.
1) Micro-barrier filtration technology
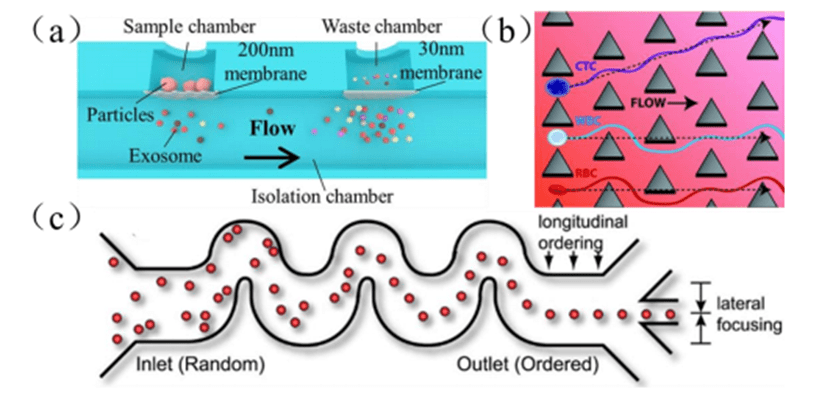
The technology utilizes porous membranes on a microfluidic platform to achieve miniaturized ultrafiltration to separate particles.
Driven by the static pressure difference, through the sieving action of the microfiltration membrane, particles smaller than the pore size pass through smoothly, while larger particles are blocked and accumulate on the surface of the membrane, forming a concentrated layer, thus realizing separation.
Micro-barrier filtration technology is similar to ultrafiltration, but is prone to clogging due to the presence of membranes, resulting in lower separation efficiency.
2) Deterministic Lateral Displacement Technique (DLD)
The technology is a hydrodynamics-based microfluidic technology that creates unique flow streamlines by placing periodically distributed micro-obstacles in microfluidic channels. The particles interact with the micro-obstacles to achieve flow manipulation.
Particles smaller than the critical size threshold continue to flow along the initial flow line, while particles larger than this threshold collide with micro-obstructions, migrate laterally to another flow line, and are eventually collected at a specific exit area to achieve separation.
The technique is simple to operate and highly accurate, but has limitations, such as a large array of micro-obstructions that limit throughput and tend to lead to clogging of the flow path.
3) Inertial Microfluidics
This technique enables particle manipulation by inducing inertial effects (e.g., inertial migration or cross-sectional Dean flow) in microfluids at finite Reynolds numbers.
Inertial microfluidics has become an important technology in the field of microfluidics for particle focusing, sorting, separation and concentration due to its simple structure, high separation efficiency, high processing throughput, high integration and no effect on cell activity.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1