Three-dimensional cell culture makes possible interactions between cells and between cells and the extracellular matrix. Tissue-like and organ-like tissues are three-dimensional miniature tissues formed by self-assembly of various cells after differentiation of pluripotent stem cells, and their structures and functions are similar to human organs.
The introduction of microfluidic technology can provide not only a mechanical microenvironment, but also simulate the biochemical and structural microenvironment of human organs that change in spatial and temporal dimensions. Meanwhile, the application of real-time monitoring technologies, such as biosensing, enables long-term non-destructive and repetitive testing of 3D models, including biophysical and biochemical signals, as well as pH and oxygen level measurements.
In drug discovery and toxicity screening, although current in vitro 3D models are still unable to completely replace animal experiments due to the limitations of the level of development, the accuracy of in vitro 3D models in some cases has surpassed that of animal experiments due to the fact that their cells are derived from humans.
1. Study of 3D-CMCs modeling
3D-CMCs (3D cell culture microarrays) is a multidisciplinary cross-disciplinary technology that integrates human physiology, tissue engineering, stem cell biology, microengineering, material science, mechanics, pharmacology and mechanics, etc. The core of 3D-CMCs is to construct three-dimensional cell models on microfluidic chips. The current commonality of microfluidic chips is mainly reflected in the material selection, region division and fabrication technology.
Ideal biomaterials should have the following properties: minimal cytotoxicity and uptake of biomolecules and drugs, be transparent to facilitate microarray imaging, have good gas permeability to ensure oxygen supply to the cells while providing structural support for 3D cells, and be able to contribute to the phenotype and function of healthy or diseased tissues.
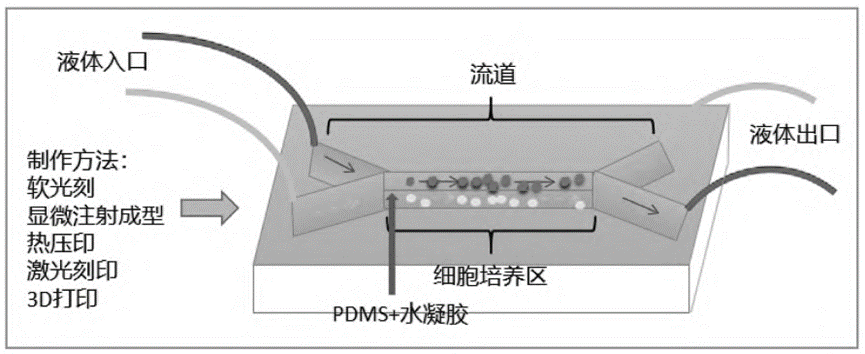
Among them, polydimethylsiloxane (PDMS) is widely used in 3D-CMCs because of its low cytotoxicity, excellent optical transparency, and permeability; however, PDMS absorbs hydrophobic small molecules or drugs from the solution. To overcome this problem, hydrogel, a hydrophilic biomaterial, is usually coated on the surface of PDMS to minimize drug absorption. Therefore, the combination of PDMS and hydrogel is very common in microfluidic chip construction.
The functional areas of microfluidic chips are basically divided in the same way, including inlet/outlet, flow channel and cell culture area. There are five common microfluidic chip fabrication techniques: soft lithography, microinjection molding, hot embossing, laser etching and 3D printing.
In constructing 3D-CMCs, 3D bioprinting technology can be used to print not only microfluidic chips as reaction vessels, but also bionic tissues as reaction receptors. Its convenience and customizability make 3D bioprinting a promising technology. By printing hydrogels, factors such as the biochemical microenvironment and oxygen concentration that change over time can be customized.
A variety of cell types can be used for the construction of 3D-CMCs models, such as representative cell lines, pluripotent stem cells, tumor cell lines and primary cells. In the 3D-CMCs system, the vascular network is of great significance to provide adequate oxygen and nutrient supply and effective control of the microenvironment for long-term tissue culture and functionalization.
Currently, the applications of 3D-CMCs are very diverse, including the construction of single-organ models such as liver, heart, blood vessels, kidney, blood-brain barrier, spleen, lung, intestines, nerves, etc., as well as combination models of blood vessels and various tissues and organs, and multiple-organ combination models, etc. 3D-CMCs models can be widely used in the fields of drug discovery and screening, organ growth and development, in-vitro lesion modeling, regenerative medicine, and drug toxicity research.
2. Application of 3D-CMCs modeling in drug toxicity testing
1) 3D-CMCs modeling in hepatotoxicity testing
Rapid high-throughput production of liver microarrays or liver tissues using 3D bioprinting technology can significantly reduce production costs and handling difficulties. Hydrogel-based liver chip systems have been developed for generating bionic liver tissue.
Some investigators have used sacrificial bioprinting with agarose and methacryloyl gelatin (GelMA) to build microchannel networks, encapsulate HepG2/C3A cells in GelMA, and implant human umbilical vein endothelial cells (HUVECs) in microchannels, thereby constructing vascular-containing liver models to assess hepatotoxicity.
In recent years, innovations in perfusion culture techniques have further simplified microarray fabrication and made it more suitable for drug toxicity testing. For example, instead of using conventional pumps, some methods enable perfusion chips with fixed flow direction through phase pointing or carbon dioxide pressure actuation. In addition, combining organoid culture with microfluidic chips allows for more precise control of the microenvironment for organoid growth.
The onset and progression of liver injury is a dynamic process that cannot be characterized by endpoint measurements alone. Combining microarrays with biosensors enables real-time monitoring of a wide range of parameters, thus providing a more comprehensive picture of liver health and its response to external stimuli.
2) 3D-CMCs modeling in cardiotoxicity testing
Currently, cardiotoxicity screening models rely on two-dimensional cell culture, animal models, and membrane clamp techniques. However, as mentioned in the introduction, 2D cell and animal models each have their limitations, while membrane clamp techniques usually require invasive manipulation. Therefore, there is an urgent need to develop three-dimensional in vitro models to more effectively detect cardiotoxicity of drugs.
A study has constructed microelectrode array chips based on human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and multi-array technology. This approach was able to generate layered cardiac tissue similar to natural myocardium and simultaneously measure electrophysiological data. By measuring the response to a cardiotoxic prodrug (terfenadine) and a non-cardiotoxic metabolite (fexofenadine), this model of 3D cell culture microarrays (3D-CMCs) was shown to be effective for drug cardiotoxicity screening.
3) 3D-CMCs modeling in nephrotoxicity testing
The researchers developed a 3D-CMCs device that mimics the kidney organ, which consists of two parts: a microfluidic drug concentration gradient generator and a flow-temperature control platform for renal cell culture. By detecting renal proximal tubular epithelial cells and peritubular capillary endothelial cells, the device was able to effectively assess drug-induced nephrotoxicity.
This 3D model demonstrated higher accuracy and performance in cell growth and drug nephrotoxicity assessment compared to conventional 2D cell culture. In addition, it was found that cimetidine intervention significantly reduced cisplatin-induced nephrotoxicity in this 3D-CMCs device, suggesting the potential of this device for studying drug interactions and toxicity assessment.
4) Application of 3D-CMCs Modeling in Combined Multi-Organ Toxicity Detection
Single-organ models aim to reconstruct key functional units of a specific organ rather than the entire organ, and thus often fail to replicate normal or abnormal interactions between different tissues and organs. In contrast, multi-organ co-chips have the advantage of being able to integrate drug metabolism and toxicity processes into a single device, facilitating a comprehensive evaluation of the toxicity of drug metabolites.
A related study reports a system that integrates multiple 3D-CMCs to evaluate drug efficacy and toxicity on multiple tissues in parallel. First, the system integrated liver, heart, and lung structures to construct a multi-organ chip and was able to maintain it under conditions of high survival for 21 days. The validity of the system was validated by the known metabolic conversion of the prodrug capecitabine to 5-fluorouracil in the liver, and by observing the toxic response of the lungs and heart to the metabolite.
The integrated system was then further expanded to accommodate liver, heart, lung, endothelial, brain and testicular-like tissues. After 14 days of incubation, interactions between multiple tissues were demonstrated by detecting downstream neurotoxicity induced by chloroacetaldehyde produced during hepatic metabolism of the prodrug isocyclophosphamide. This system demonstrates a new way of assessing drug effects and toxicity in a multi-organ setting with important application potential.
Site Search
© 2025. All Rights Reserved. 苏ICP备2022036544号-1













