A common application strategy for microfluidic chips is to machine micrometer to sub-millimeter fluidic channels or detection chambers on the surface of the chip substrate. These microstructures are usually closed by bonding techniques and coupled with fluid pumping devices and detection and analysis devices to automate fluid delivery and results analysis.
Currently, there are two main forms of microchannel structures for microfluidic chips:
1. Sheet-like laminated structures: Microfluidic interactions in such structures occur in a two-dimensional plane. Common materials include PDMS, PMMA and glass.
2. Three-dimensional coaxial tubular nested structures: These structures allow fluids to interact with each other in three dimensions and are commonly represented by capillary microfluidic chips.
Capillary microfluidic chips offer a variety of advantages: their unique three-dimensional symmetric fluidic channels allow microfluidics to interact in three-dimensional space, making it easier to achieve effects such as droplet or fiber generation.
Since the specific fluid does not directly contact the channel wall, it can effectively prevent microchannel clogging. Compared with PDMS and PMMA microfluidic chips, capillary microfluidic chips do not require complex microfabrication techniques such as photolithography, which dramatically reduces fabrication costs and simplifies the fabrication process.
Glass capillary tubes have high hardness, heat resistance and pressure resistance, and can be used in high-pressure ionization and other fields. In addition, the chemical stability of the capillary tube is good, not easy to dissolve and chemical corrosion resistance, the common organic reagents have a strong resistance, suitable for a variety of substances transport or as a micro-reactor, a wide range of applications.
1. Capillary microfluidic chip-based droplet generation methods
1) Capillary Microfluidic Chip Droplet Generation Principle
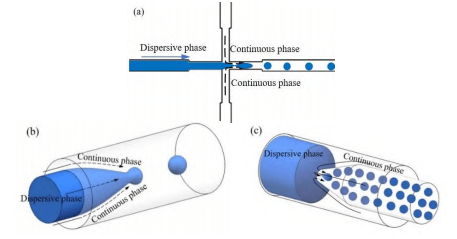
There are two main ways of capillary droplet generation: two-phase co-flow and counter-current focusing. In the two-phase co-flow type, the continuous phase fluid and the dispersed phase fluid flow in the same direction.
Since the size of the dispersed phase outlet end is smaller than the size of the channel, a certain pressure gradient is formed at the outlet, and the dispersed phase fluid is gradually thinned by the extrusion and shear of the surrounding annular continuous phase fluid. When the surface tension at the liquid-liquid interface is not enough to balance the shear force, the dispersed phase fluid will break and form droplets.
In the countercurrent focusing type, the continuous phase fluid flows in the opposite direction to the dispersed phase fluid. The dispersed phase fluid forms droplets under the uniform shear force of the continuous phase fluid in the opposite direction.
2) Application of capillary microfluidic chip in the direction of droplet generation
a) Single encapsulated droplets
One of the main functions of capillary microfluidic chips is to generate microdroplets. Through different capillary nesting methods, it was found that countercurrent focusing was more likely to generate smaller-sized droplets compared to co-current focusing in the experiments of preparing poly(lactic acid) (PLA) and polycaprolactone (PCL) nanoparticles.
In the preparation of monodisperse droplets by countercurrent focusing, different droplet generation modes can be explored by adjusting the two-phase flow rate ratio and the inner diameter of the conical pore.
This process can be used to prepare PLA and polylactic acid-hydroxyacetic acid copolymer (PLGA) particles with tunable size and frequency, which provides an experimental basis for realizing controlled drug release in the human body.
b) Double wrapped droplets
By varying the nesting and inlet orientation of the glass tubes, multiple types of double-wrapped droplets can be generated using either a one-step or a tandem two-step method. Using the one-step method, the researchers prepared polystyrene target spheres, laying the groundwork for future laser fusion experiments.
In addition, the use of photopolymerization to encapsulate phase change materials in microcapsule shells improves the encapsulation rate and provides a new solution for the storage of phase change materials.
Typically, double-encapsulated droplets prepared in a one-step method are mostly core-shell droplets. In contrast, the researchers have successfully prepared double-encapsulated droplets containing multiple monodisperse internal droplets by using a two-step method in tandem.
By adjusting the fluid flow rate, fluid formulation, and geometry of the microstructure, it is possible to precisely control the number of internal droplets and the density of buildup in the outer droplets.
c) Multi-parcel generation of droplets and droplet slicing
Multi-encapsulated microcapsules typically contain multiple reagents, enabling sequential release of multi-component drugs in biotherapeutics while avoiding cross-contamination.
As different sizes of capillaries can flexibly realize multiple nesting modes, the microfluidic device formed by the assembly of different capillaries can also be used for the preparation of multiply wrapped and multinucleated wrapped microcapsules.
On this basis, the splitting of droplets can also be realized, providing a new technical means for droplet size control and mass production.
In addition, by combining tapered-mouth glass capillaries with 3D printing technology, Janus microdroplets and magnetically responsive microdroplets can be prepared, making capillary microfluidic chips promising for applications in sensors and actuators.
2. Capillary microfluidic chip-based spinning methods
1) Ionic Crosslinking
The basic principle of generating microfibers by ionic cross-linking is that sodium alginate rapidly undergoes ion exchange when it comes into contact with calcium ions, rapidly forming a gel. A variety of forms of microfibers can be generated using multiple nesting of capillaries.
In addition, glass capillaries were inserted into PDMS and microfluidic chips were fabricated by plasma bonding, which enabled microfluidic wet spinning of chitosan-sodium alginate ultrafine fibers and encapsulation of HepG2 cells.
By changing the nesting of capillaries, hollow fibers of sodium alginate can also be generated to introduce cells, enzymes, etc. into the hollow fiber tubes, which has important applications in microvascularized tissue engineering and biocatalytic microchemical reactors.
2) Ultraviolet polymerization
The basic principle of generating microfibers by UV polymerization is that when a capillary fluid containing a photoinitiator is irradiated with UV light (usually 365 nm), rapid curing is achieved through polymerization and cross-linking reactions.
In the preparation of microfibers, UV polymerization is usually coupled with ionic cross-linking. Taking advantage of the rapidity of UV polymerization, microfibers containing spindle junctions or cells are prepared with higher controllability in size and arrangement, providing an experimental basis for the application of microfibers in biological tissue structures.
By inserting glass capillaries into PDMS, the assembled microfluidic chip is able to rapidly cure microfibers by UV polymerization, which not only achieves the immobilization of enzymes in fibers, but also prepares PLGA microfibers for generating three-dimensional tissue engineering scaffolds.
3) Solvent exchange method
The basic principle of the solvent exchange method for generating microfibers is that by allowing the solvent in the dispersed phase to diffuse freely into the outer phase solution, components such as polymers in the dispersed phase are solidified and collected as the solution flows.
Helical and superhelical structures are critical in achieving tissue function. Helical and superhelical microfibers can be continuously and controllably generated using microfluidic chips composed of capillary nests.
By adjusting the flow rate and flow velocity of each phase, the size parameters of these microfibers can be precisely controlled to prepare microfibers of different specifications. These microfibers exhibit excellent tensile properties in magnetically responsive elastic microactuators and have promising applications.
3. Other applications of glass capillary microfluidic chips
In addition to the above applications, the combination of optical and microfluidic technologies has brought many innovations. For example, by using capillary microcavities as fluid channels and utilizing the ring resonator structure of its wall cross-section, it is possible to realize low-threshold, wavelength-tunable optical flow lasers on microfluidic chips by using spatial optical pumping, for example, as a pumping source.
In addition, the detection of biochemical liquid-phase samples is also possible by utilizing the high-quality factor optical resonance cavities (WGMs) of silica glass capillaries and good refraction.
In mass spectrometry, microfluidic chips are coupled with mass spectrometry for rapid detection of trace amounts of samples in order to reduce the amount of samples used, shorten the analysis time, and reduce costs.
For example, mounting a capillary or spray needle at the end of a microfluidic chip flow channel made of PDMS enables more stable results than direct spraying, with less solution consumption, lower voltage required, and a significant increase in detection speed. This method can be used for the separation and detection of chemicals in amino acid mixtures and nerve cells.
Capillary tubes are often used as a storage or delivery device for samples or reagents due to their smooth inner walls and excellent surface properties. By attaching a polyimide-coated glass capillary to a ceramic plate, it can be used as an actuator to manipulate droplets in the capillary.
Capillary glass tubes can also be used as sample or reagent reservoirs, and microfluidic enzyme-linked immunosorbent assay (ELISA) devices formed by arrays are able to reduce analytical testing time and sample usage, and are widely used for rapid biochemical analyses in biomedical and R&D laboratories.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1