In recent years, microfluidic platforms have been used in a wide range of cellular analyses, such as manipulating single cells, automating media perfusion, providing cellular microenvironments and external stimuli to study cellular responses, and establishing long-term cell culture systems.
With the development of microfluidic chip technology and its application characteristics, it has become a trend to utilize microfluidic chip technology for Alzheimer's disease (AD)-related research.
The main neuropathological features of AD include beta amyloid (Aβ) plaque deposition, phosphorylated Tau protein (p-Tau), Tau protein aggregates in neuroprogenitor fibril tangles (NFTs) and neuronal protrusions, cerebral cortical atrophy due to synaptic deficits and neuronal loss, astrocyte and microglia activation, neuroinflammation, and neurological damage.
In addition, factors affecting the pathogenesis of AD include functional lesions of the cardiovascular and lymphatic systems of the brain, aging, the blood-brain barrier (BBB), the peripheral immune system, and intestinal flora.

Reported AD pathology models mainly include animal models and in vitro cellular models, which usually reproduce one or more of the AD pathophysiological features. Ideal animal models of AD are important in the discovery of therapeutic targets, the unraveling of AD pathogenesis, and the development of anti-AD drugs.
The AD cell model is a relatively simplified platform for observing and testing the pathological and biological processes of AD under controlled conditions, and is important for studying the biological functions of proteins associated with AD pathogenesis.
Cells used to construct AD cell models mainly include astrocytes, microglia, endothelial and neuronal cells, induced pluripotent stem cells (iPSC), human brain microvascular endothelial cells (HBMEC, HCMEC), neural stem cells, and neuroblastoma cells (SH-SY5Y).
Construction of a microfluidic chip AD pathology model
Traditional animal models and in vitro cellular models have limitations in the study of AD. Constructing AD models with a quasi-physiological environment in vitro using microfluidic chip technology provides a unique solution for AD research.
The AD microfluidic chip model mainly includes elements such as chip structure, cell culture and factors inducing AD pathological features. The model construction simulates the structure and function of the brain by selecting cell types, performing cell 3D culture and designing the chip structure, and investigates the complex interactions between the brain microenvironment and the neurovascular unit.
Cell type
Modeling AD pathology requires an understanding of the cell types of the functional units of the brain, the composition of the internal environment, and their interactions.
Using cells associated with the pathogenesis and pathological features of AD, such as neurons, astrocytes, microglia, brain endothelial cells, and cells in the blood-brain barrier (BBB), extracellular matrix or scaffolds are added, and these cells are inoculated into a microfluidic chip system.
After a period of culture, the relevant factors that induce and express the pathological features of AD were added to gradually form a more complete AD microfluidic chip model.
By combining organ-on-a-chip technology and brain microvascular endothelial cells, astrocytes and neurons obtained by differentiation of human iPSCs, a neurovascular unit capable of encapsulating complex BBB functions has been constructed, which provides a research platform for modeling BBB-associated diseases, investigating the roles of the BBB, drug screening, and individualized medicine.
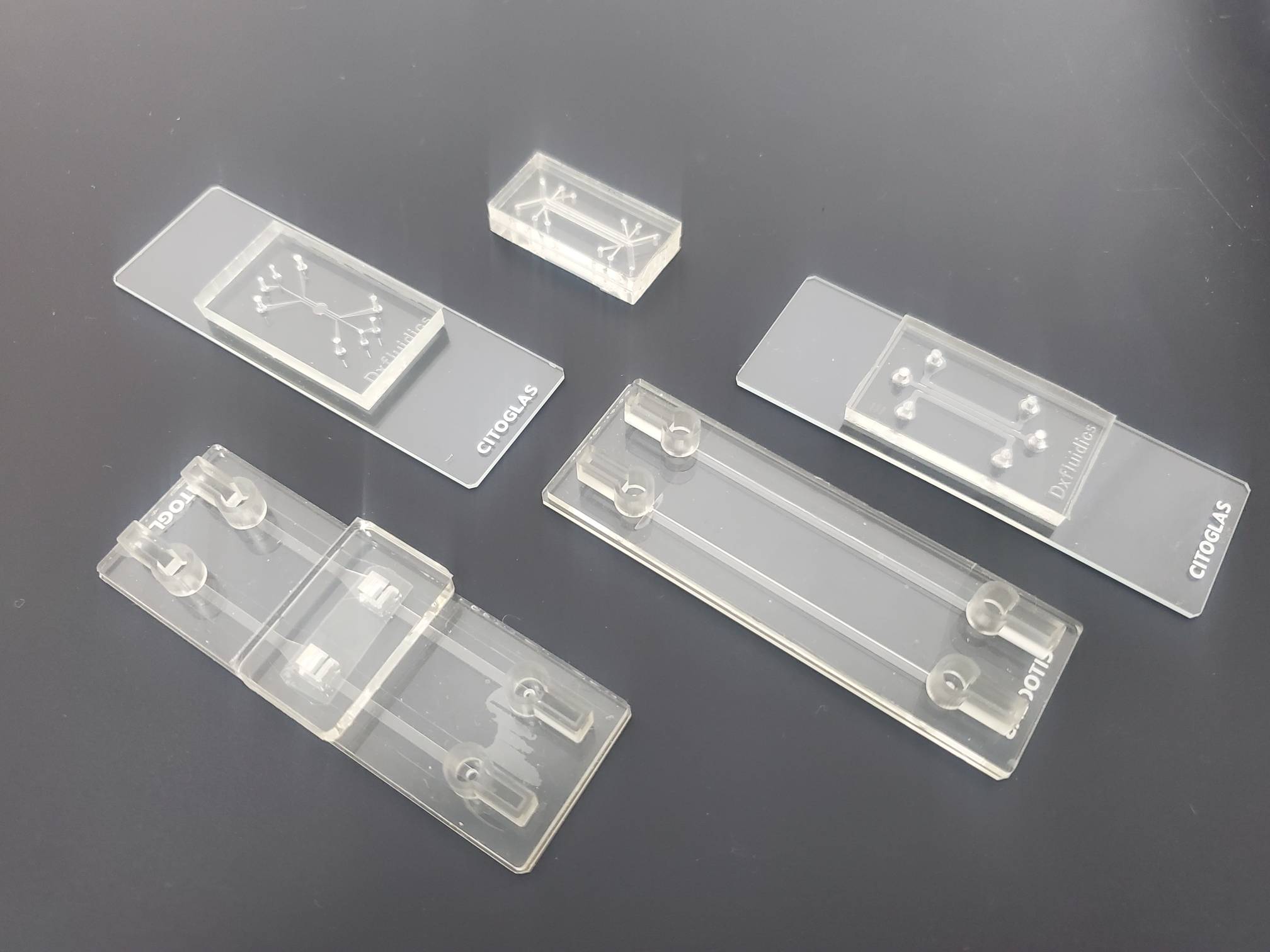
Cell 3D culture model
2D cell culture is one of the most commonly used models for assessing different disease systems, but most 2D cultured AD cell models lack the essential features of cell-to-cell contacts and interactions in 3D brain tissue.
3D culture can mimic in vitro cell morphology, cell attachment, proliferation, response to stimuli, differentiation, drug metabolism, and intercellular flow in vivo.
The extracellular matrix and biochemical effects of 3D cultures provide a level of mechanical strength and integrity that maintains the dynamic equilibrium of neural regions, forming highly complex networks that play a key role in regulating the processes of neural stem cell differentiation, neuronal migration, and the maturation and function of central and peripheral synapses.
AD models that have been reported for in vitro 3D culture include neurosphere systems, microfluidic chip culture systems, organoid culture systems, 3D hydrogel systems, 3D bioprinting systems, and in vitro bioengineered models of neurovascular units.
Among them, the 3D culture mode based on microfluidic chip technology can mimic the in vivo microenvironment at the cell, organ or multi-organ level, which makes it possible to cultivate neurons sensitive to the cell culture environment for a long period of time, and provides a more reliable platform for the study of neuronal interactions, functions and signaling pathways in AD.
chip structure
3D co-culture based chip structures
The co-culture model allows for the study of the interaction of two or more cell types.
Conventional 2D co-culture systems are spatially incapable of accurately localizing cells or cell parts (e.g., axons and cytosol of neurons) or selectively analyzing and probing specific cells.
Using microfluidic chip technology it is possible to design compartment-specific pathology models for 3D culture and co-culture conditions that mimic AD pathology characteristics.
The first 3D co-culture microfluidic chip AD model A physiologically relevant 3D neurosphere microfluidic brain chip model was constructed by co-culturing tubular brain endothelial cells with 3D differentiated ReN-AD cells via a microfluidic chip with concave microwell arrays and a micro-osmotic pump system.
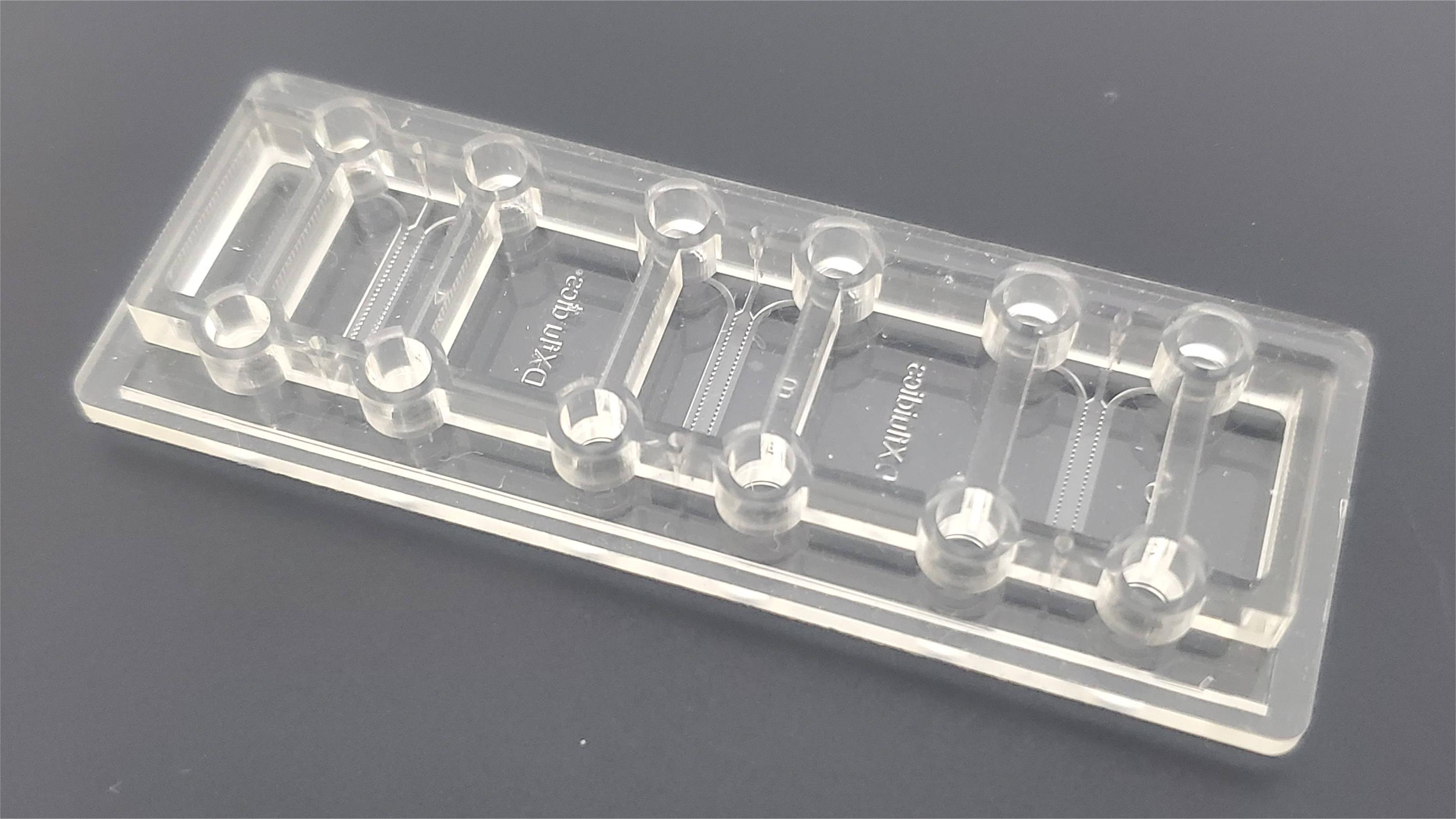
The microarray model can simulate the interstitial level flow of the in vivo microenvironment and perform long-term in vitro observation without other auxiliary equipment, which has good application prospects for studying the pathology and therapeutic strategies of AD or other neurological diseases.
Chip structure based on blood-brain barrier unit
The blood-brain barrier (BBB) is the primary barrier to the entry of endogenous substances and therapeutic drug molecules into brain tissue.The highly selective permeability of the BBB arises from its unique structure, which consists of endothelial cells arrayed within the vasculature, pericytes associated with the basement membrane, structures that are tightly connected to the endothelium, and astrocyte pedicles that extend their ends to the proximal luminal side of the vessel.
In addition, the expression of exocytosis pumps in BBB endothelial cells prevents the diffusion of small molecules across cell membranes.The BBB not only protects neural tissues from toxins in the bloodstream, but also restricts the passage of therapeutic drugs. Its complexity also makes it difficult to develop models that mimic its health state and disease characteristics.
In vivo models (animal models), 2D cellular models and in vitro 3D models of the BBB have been studied.
Among them, BBB models such as in vitro 3D culture Transwell model and microfluidic chip model constructed using relevant cell lines provide more physiologically relevant models for research across the BBB and drug development for CNS diseases.
Application of microfluidic chip technology in AD research
Compared with traditional in vitro culture systems, microfluidic chips have significant advantages in simulating typical tissue complexity features such as brain multicellularity, 3D culture, vascularization, and extracellular matrix, and are often used to simulate and study physiological phenomena in the central nervous system, such as neural network development, intercellular signaling, and immune function.
The microfluidic chip can also be designed as a multifunctional platform integrating model construction, drug efficacy and potential adverse reaction evaluation.
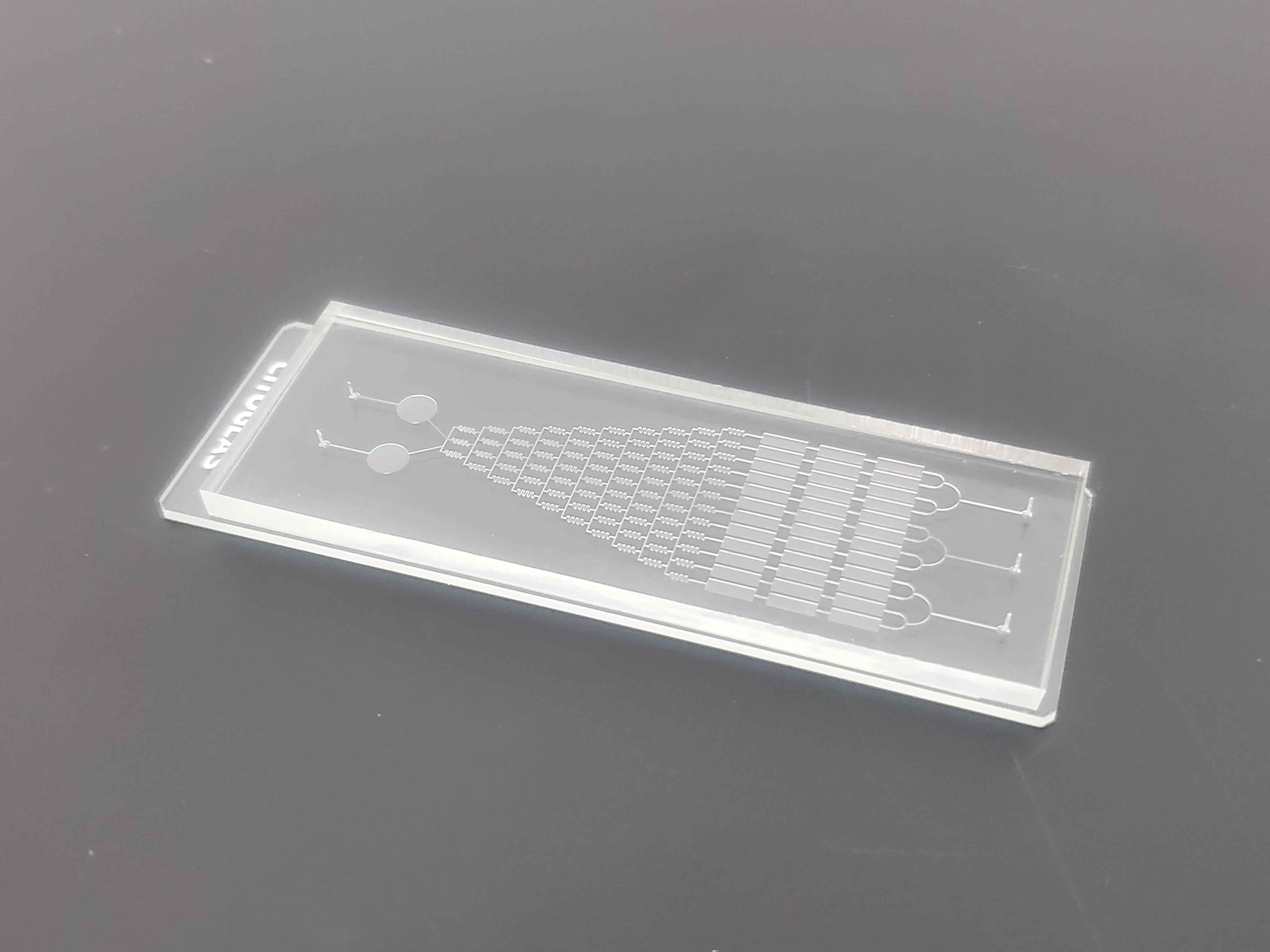
Based on these advantages, microfluidic microarrays can develop more physiologically relevant in vitro models, partially replacing animal studies. In recent years, microfluidic chips have been gradually applied to the study of pathological mechanisms of AD, early diagnosis and drug screening.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1













